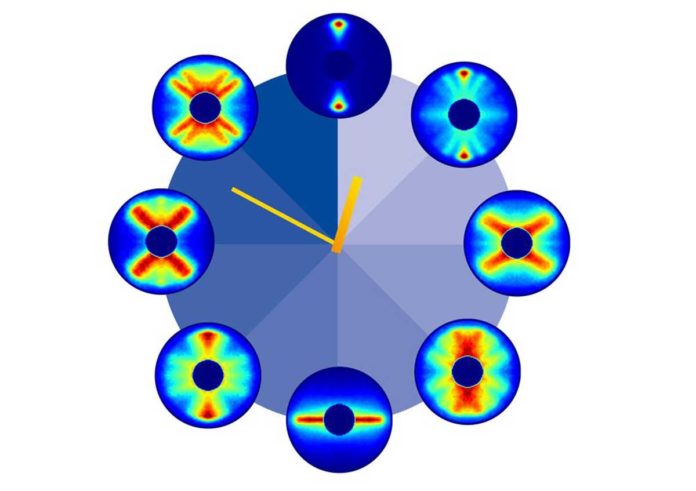
Using precisely tuned laser light pulses, scientists at the have been able to film the ultrafast rotation of a molecule. They have filmed a molecular movie, in which they tracked one and a half rotation of carbonyl sulfide (OCS)- taking place within 125 trillionths of a second, at a high temporal and spatial resolution.
Molecular physics has since a long dreamed of capturing the ultrafast motion of atoms during dynamic procedures on film. Although, this is not easy as there is a need for high-energy radiation in the realm of molecules.
Thus, scientists used another approach: By using two pulses of infrared laser light which were correctly tuned to one another and isolated by 38 trillionths of a second (picoseconds), to set the carbonyl sulfide molecules spinning quickly in unison.
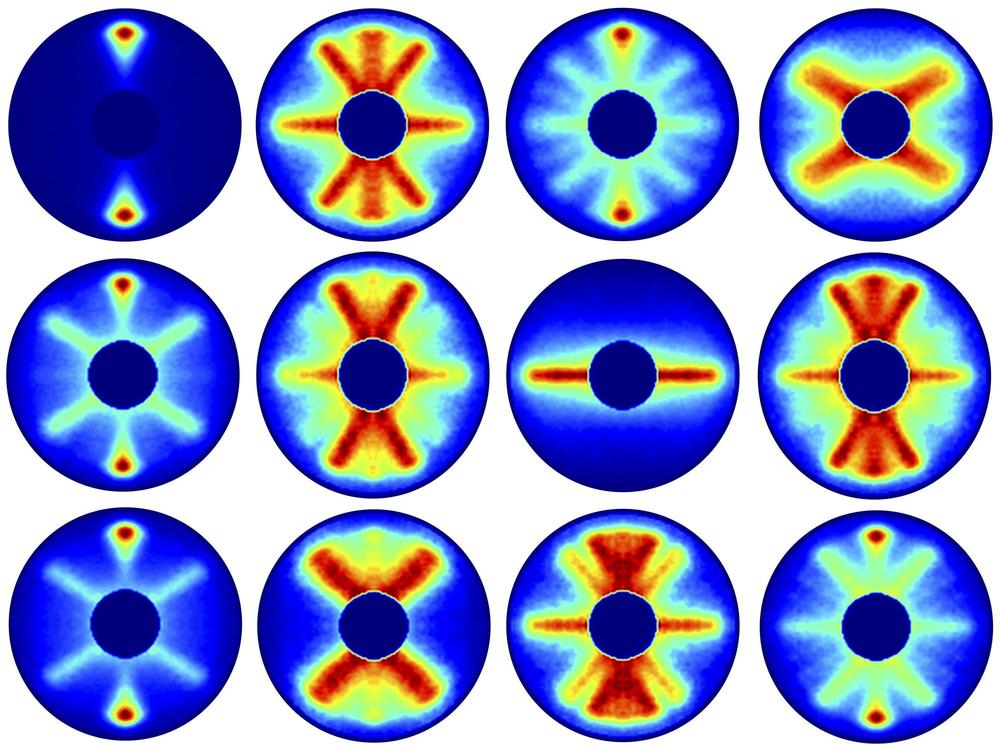
They then used a further laser pulse, having a longer wavelength, to determine the position of the molecules at intervals of around 0.2 trillionths of a second each.
Evangelos Karamatskos, the principal author of the study from CFEL, said, “Since this diagnostic laser pulse destroys the molecules, the experiment had to be restarted again for each snapshot.”
Scientists captured 651 pictures covering one and a half periods of rotation of the molecule. Gathered consecutively, the photos created a 125 picosecond film of the molecule’s rotation. The carbonyl sulfide atom takes around 82 trillionths of a second, for example, 0.000 000 082 seconds, to finish one entire revolution.
DESY´s Jochen Küpper from the Center for Free-Electron Laser Science (CFEL) said, “It would be wrong to think of its motion as being like that of a rotating stick, though. Quantum mechanics governs the processes we are observing here. On this scale, tiny objects like atoms and molecules behave differently from the everyday objects in our surroundings. The position and momentum of a molecule cannot be determined simultaneously with the highest precision; you can only define a certain probability of finding the molecule in a specific place at a particular point in time.”
Arnaud Rouzée from the Max Born Institute in Berlin said, “The peculiar features of quantum mechanics can be seen in several of the movie´s many images, in which the molecule does not simply point in one direction, but in various directions at the same time – each with a different probability.”
“It is precisely those directions and probabilities that we imaged experimentally in this study. From the fact that these individual images start to repeat after about 82 picoseconds, we can deduce the period of rotation of a carbonyl sulfide molecule.”
Evangelos Karamatskos, the principal author of the study from CFEL said, “The scientists believe that their method can also be used for other molecules and processes, for example, to study the internal twisting, i.e., torsion, of molecules or chiral compounds, that are compounds that exist in two forms, which are mirror images of each other – much like the right and left hands of a human being.”
“We recorded a high-resolution molecular movie of the ultrafast rotation of carbonyl sulfide as a pilot project. The level of detail we were able to achieve indicates that our method could be used to produce instructive films about the dynamics of other processes and molecules.”
The results of the study are published in the journal Nature Communications.