Stem Cells are the clean slate on which every specific cell in our bodies are manufactured and they are the foundation for each organ and tissue in the body.
It has been over 10 years since researchers initially demonstrated that mature cells can be reinvented in the lab to wind up pluripotent stem cells that are fit for being produced into any cell type in the body. In those early examinations, analysts hereditarily altered mature cells by presenting external factors that reset the genomic projects of the cells, essentially turning back the clock and returning them to an undifferentiated or unspecialized state.
The resultant lab-made cells, known as induced stem pluripotent stem cells (iPSCs) would then be able to be customized into various cell writes for use in tissue repair, drug discovery and even to develop new organs for transplant. Importantly, these cells did not to be harvested from embryos.
Recent research led by Professor G.V. Shivashankar of the Mechanobiology Institute (MBI) at the National University of Singapore (NUS) and the FIRC Institute of Molecular Oncology (IFOM) in Italy, has revealed that mature cells can be reprogrammed into re-deployable stem cells without direct genetic modification – by confining them to a defined geometric space for an extended period of time.
A major obstacle is a tendency for any specialized cell that is developed from iPSCs, to form tumors after being introduced into the body. To understand why this occurred, researchers turned their focus to understanding how stem cell differentiation and growth is regulated in the body, and in particular, how cells naturally revert to an immature stem cell-like state or convert to another cell type, during development, or in tissue maintenance.
Prof Shivashankar said, “Our breakthrough findings will usher in a new generation of stem cell technologies for tissue engineering and regenerative medicine that may overcome the negative effects of genomic manipulation.”
“The research has shown that mature cells can be reprogrammed, in vitro, into pluripotent stem cells without genetically modifying the mature cells, simply by confining the cells to a defined area for growth.”
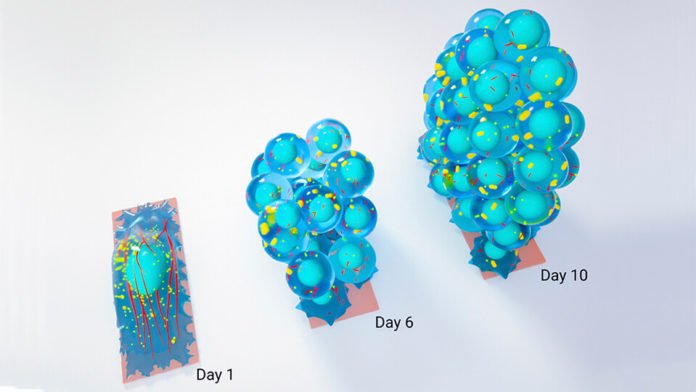
At the point when fibroblast cells were restricted to rectangular territories, they immediately expected the state of the substrate (the surface or medium that the cells are joined to). In view of past work from the Shivashankar lab, this demonstrated the cells were estimating and reacting to the physical properties of their environment, and passing on this data to the nucleus where DNA bundling and genome programmes would adjust as needs be.
The group developed the cells more than 10 days until the point that they shaped spherical clusters of cells. Genetic analysis of the cells within these clusters revealed that specific characteristics of chromatin (the condensed form of packaged DNA) normally associated with mature fibroblasts were lost by the sixth day.
By the tenth day, the cells expressed normally regularly connected with stem cells and iPSCs. The scientists have now learned that by limiting the develop cells for an expanded timeframe, develop fibroblasts can be transformed into pluripotent stem cells.
To affirm that the fibroblasts had in fact been reconstructed into stem cells, the scientists at that point coordinated their development, with high proficiency, into two diverse particular cell types. A few cells were likewise coordinated over into fibroblasts.
The physical parameters used in the study are reflective of the transient geometric constraints that cells can be exposed to in the body. For example, during development, the establishment of geometric patterns and niches are essential in the formation of functional tissues and organs. Similarly, when tissue is damaged, either through injury or disease, cells will experience sudden alterations to their environment. In each case, mature cells may revert back to a pluripotent, stem cell-like state, before being redeployed as specialized cells for the repair or maintenance of the tissue.
Prof Shivashankar explained, “While it is well established that confining stem cells to defined geometric patterns and substrate properties can direct their differentiation into specialised cells, this study shows for the first time that mechanical cues can reset the genomic programmes of mature cells and return them to a pluripotent state.”
“The use of geometric constraints to reprogramme mature cells may better reflect the process occurring naturally within the body. More importantly, our findings allow researchers to generate stem cells from mature cells with high efficiency and without genetically modifying them.”
The team’s research findings were published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS) in May 2018.