For a long time, it was considered that these interactions between molecules are always attractive. Now, for the first time, found that in many rather common situations in nature the Van der Waals force between two molecules becomes repulsive.
Introduced in 1930, Van der Waals force is a term that defines the attraction of intermolecular forces between molecules. It only pushes back when groups of molecules are under pressure. According to the new research, such reversal can occur in the real world where crowds of molecules jostle freely.
Scientists applied a model that shows how the charges on particles become polarized under certain conditions. They then compared their findings with experimental results and found that the Van der Waals force can push when they were only expected to pull.
Electrons as negatively charged particles that move around a positively charged nucleus. While moving, they are more likely to occupy some areas around the atom more than others. It depends on what else happens to be pushing and shoving nearby.
Additionally, particles of the same charge have a repulsive effect on one another. Thus, electrons bunch up and make that part of a molecule more negative. They next pull towards the zone with more positive molecules.
Such loose bonding makes H2O molecules more sticky. This gives the liquid a high surface tension that makes belly flops in the swimming pool hurt so much.
Van der Waal forces are like these so-called hydrogen bonds. The force is only applied when the molecules are not so imbalanced. Although, Van der Waal forces are also not that much powerful than other forms of chemical bonding. They also require molecules to be relatively close to one another.
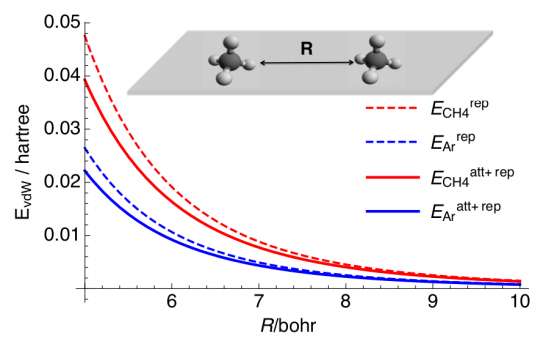
And squeezing molecules close together can shift their electron charge density. The researchers wanted to know if the same rearrangements of electrons could sometimes be repulsive under other crowded conditions that weren’t under high pressure.
Alexandre Tkatchenko, a researcher from the University of Luxembourg said, “The textbooks so far assumed that the forces are solely attractive. For us, the interesting question is whether you can also make them repulsive.”
To identify, scientists used a model called a Drude oscillator. The model shows the fluctuating charge densities around particles in a confined space. It demonstrates the tiny tugs between molecules over short distances. Sometimes it turns the molecules into the occasional shove, even when they’re not being squeezed together.
Researcher and developer of the model, Mainak Sadhukhan said, “We could rationalize many previous experimental results that remained unexplained until now. Our new theory allows, for the first time, for an interpretation of many interesting phenomena observed for molecules under confinement.”