The early detection of the onset of transplant rejection is critical for the long-term survival of patients. The diagnostic gold standard for detecting transplant rejection involves a core biopsy, which is invasive, has limited predictive power and carries a morbidity risk.
In a new study by the Georgia Institute of Technology, scientists have developed a novel approach using sensor particles and a urine test. The method could potentially catch rejection much earlier, more comprehensively, and without a biopsy needle.
Scientists validated the method in a mouse model, and they have built the sensor with exceptionally biocompatible parts, which could make the way to potential future trials easier.
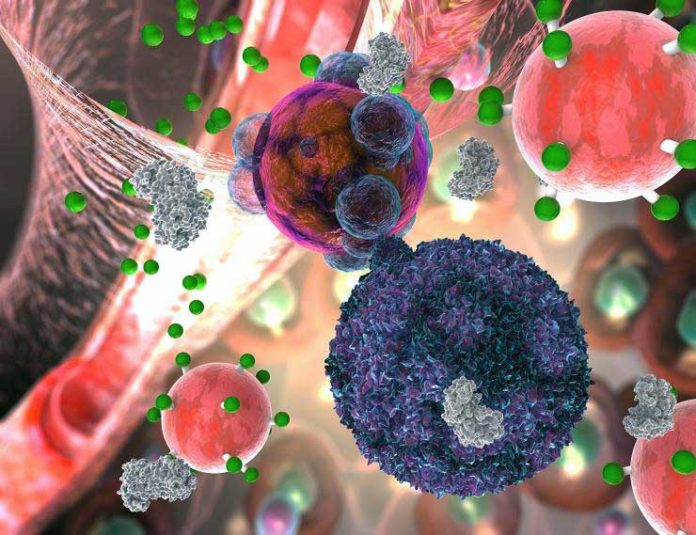
A patient may feel fine, and a biopsy may look misleadingly perfect when T cells have just started assaulting a transplanted organ. The sensor molecule, a nanoparticle, detects a T cell weapon, a catalyst called granzyme B, that pushes a transplanted organ’s cells into the self-destruction procedure called apoptosis.
Gabe Kwong, a co-principal investigator in the study and an assistant professor in the Wallace H. Coulter Department of Biomedical Engineering at Georgia Tech and Emory University said, “Before any organ damage can happen, T cells have to produce granzyme B, which is why this is an early detection method.”
“This is sensitive enough to possibly detect budding rejection before you see the significant injury to the transplanted organ and that could help clinicians treat early to prevent damage. Right now, most tests are aimed at organ dysfunction, and sometimes they don’t signal there is a problem until organ function is below 50 percent.”
To develop this strategy, scientists put nanoparticles together with iron oxide in the middle like a ball. They then double coated with dextran, a sugar, and polyethylene glycol, a typical fixing in intestinal medicines, to shield the body from disposing of it too rapid.
Bristles made of amino acids stand out from the iron ball with fluorescent reporter particles appended to their tips.
The particles are infused intravenously. They are too huge to gather in native tissue or to go through the kidneys and out of the body however little enough to aggregate in the tissue of battling transplanted organs, where they keep a post for dismissal.
Kwong said, “Once T cells start secreting granzyme B, it severs amino acid strands in the transplanted organ’s cells, triggering the cells to unravel and die.”
“The nanoparticles’ bristles mimic granzyme’s amino acid targets in the cells, so the enzyme cuts the bristles on the nanoparticle at the same time. That releases the reporter molecules, which are so small that they easily make it through the kidney’s filtration and go into the urine.”
“In the experiment, the animals’ urine glowed and could be seen in their bladders in near-infrared images.”
Scientists are planning to augment their method to identify the other major cause of transplant rejection, attacks by antibodies, which are not living cells but proteins the body creates to neutralize foreign entities.
Kwong said, “Antibodies kill their target cells through similar types of enzymes. In the future, we envision a single sensor to detect both types of rejection. This method could be adapted to tease out multiple problems like rejection, infection or injury to the transplanted organ. The treatments for all of those are different, so we could select the proper treatment or combination of treatments and also use the test to measure how effective treatment is.”
Dr. Andrew Adams, co-principal investigator and an associate professor of surgery at Emory University School of Medicine said, “Biopsies are currently the gold standard in detection but they can go wrong, and the wide, long needle can damage tissue. The biggest risk of a biopsy is bleeding and injury to the transplanted organ.”
“Then there’s the possibility of infection. You’re also just taking a tiny fraction of the transplanted organ to determine what’s going on with the whole organ, and you may miss rejection or misdiagnose it because the needle didn’t hit the right spot. The urine test gets a more global reading on the whole organ, and it has other advantages over biopsies.”
Kwong said, “The biopsy is not predictive. It’s a static snapshot. It’s like looking at a photo of people in mid-jump. You don’t know if they’re on their way up or on their way down. With a biopsy, you don’t know whether the rejection is progressing or regressing. Our method measures biological activity rates, and that tells us where things are going.
Kwong and Adams published the study’s results in the journal Nature Biomedical Engineering on February 18, 2019.