The deep Earth’s carbon cycle accounts for about 90% of the whole carbon cycle. Despite this, We are unaware of the cycle happening beneath the Earth’s surface. This phenomenon is crucial to life on our planet as it allows carbon in the deep Earth to get back to the atmosphere.
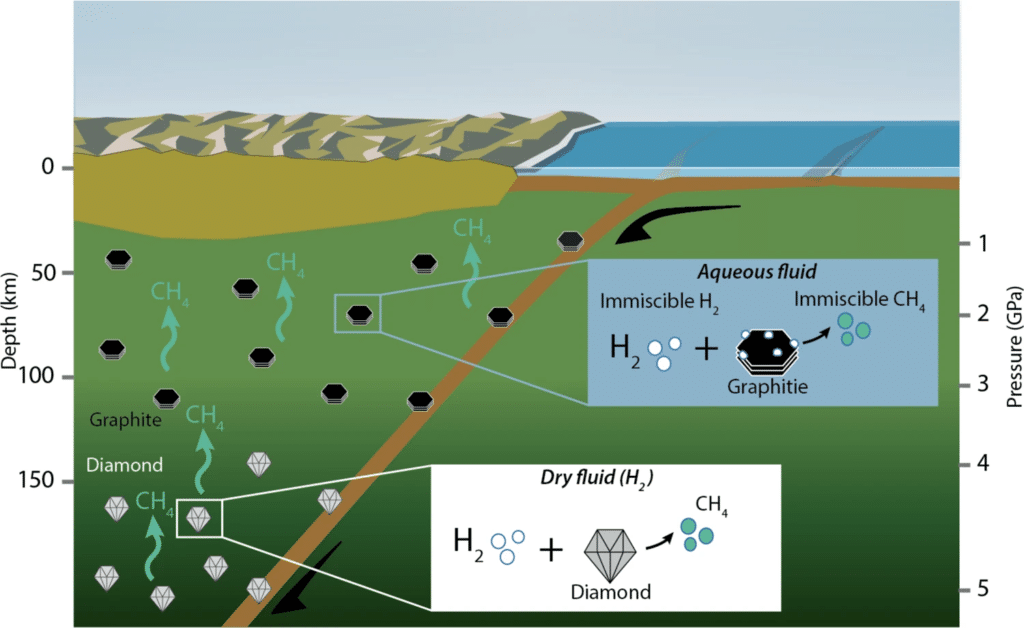
Diamond and graphite are fundamental carbon sources in the upper mantle, whereas hydrogen is among the most volatile fluid elements.
Methane at depths could co-exist with molecular hydrogen and small amounts of light hydrocarbons and different carbon allotropes. However, the origin of methane in the upper mantle remains largely unconstrained.
A group of researchers from the Universities of Bologna and Edinburgh (UK), the Centre National de la Recherche Scientifique (France), and HPSTAR (China) has unexpectedly found that high reactivity of carbon allotropes with hydrogen in Earth’s upper mantle could lead to the formation of methane. This outcome was published in Nature Communications.
This study might provide more insight into the deep carbon cycle and the formation of hydrocarbons through abiotic processes in the deep Earth.
“It is well known that the decomposition of methane may bring to diamond formation. What was less known until now is that the opposite process is also possible. Methane produced through the reaction between diamonds and hydrogen was the missing piece to a wider understanding of the deep carbon cycle”, explains Alberto Vitale Brovarone who is a professor a the Department of Biological, Geological and Environmental study at the University of Bologna and also one of the authors of the study.
The deep carbon cycle also includes the formation of hydrocarbons such as methane as the result of processes that do not involve biological activities. This theory has been under discussion for over a century. Wanting to test this theory, researchers started from diamonds that are essentially gems in the Earth’s mantle composed of solid carbon atoms in a crystal structure.
Scholars used a “diamond anvil cell,” which is a high-pressure experimental apparatus used to press two diamond culets against each other and replicate the pressure conditions of the Earth’s upper mantle, over 70 km deep. Then, by pushing one atmosphere of pure hydrogen at 300 °C, researchers observed methane rapidly forming with its molecules composed of one atom of carbon and four hydrogens (CH4).
“We created an environment comparable to that of the outer layer of earth’s mantle in terms of temperature and pressure and observed that diamond and hydrogen reacted readily by producing methane within a few seconds,” states Vitale Bovarone. “This shows that hydrocarbons such as methane can form at abiotic depths. This phenomenon may play a key role in the deep Earth’s carbon cycle”.
Researchers replicated this experiment by adding graphite, composed of pure carbon, and a glass-like carbon material. In both cases, they observed methane forming more rapidly and copiously compared to when they used only diamonds. These results suggest that carbon-based graphitic materials may be very efficient reagents and can therefore act as sources of energy feeding methane reserves in the Earth’s upper mantle.
This study was published in Nature Communication under “In-situ abiogenic methane synthesis from diamond and graphite under geologically relevant conditions.” The authors of this study are Miriam Peña-Alvarez, Mengnan Wang and Eugene Gregoryanz from Edinburgh University (UK), Mary-Ellen Donnelly, Philip Dalladay-Simpson and Ross Howie from HPSTAR – Center for High-Pressure Science and Technology Advanced Research (China) and Alberto Vitale Brovarone, a professor at the Department of Biological, Geological and Environmental Sciences of the University of Bologna.
Journal Reference
- Peña-Alvarez, M., Brovarone, A.V., Donnelly, ME. et al. In-situ abiogenic methane synthesis from diamond and graphite under geologically relevant conditions. Nat Commun 12, 6387 (2021). DOI: 10.1038/s41467-021-26664-3
