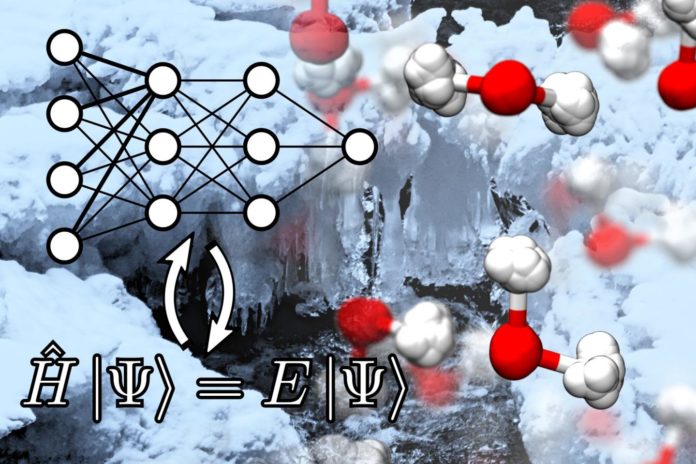
Electrons and nuclei are the building blocks of most observable matters. Their behaviors in terms of wave function can be analyzed via laws of quantum mechanics.
By solving the Schrodinger equation, it is possible to make models and predictions of any material, including water. But there is a catch. As the number of electrons and nuclei increases, the complexity involved soon become intractable even with the fastest supercomputers, and even after a century of celebrated progress in optimizing such calculations.
Yet, for systems with more than a few hundred atoms, such calculations are still unaffordable. They become imprecise when it comes to a time period longer than a nanosecond, which is 1/1,000,000,000th of a second.
In order to avoid such harsh limitations, scientists have now used an artificial neural network (ANN) to learn the atomic interactions from quantum mechanics. The ANN first studies quantum mechanical interactions between atoms, and then make speedy predictions about the energy and forces for a system of atoms.
This bypasses the need to perform expensive quantum mechanical calculations.
It can be represented as several layers of interconnected nodes, which mimics the structure of the neurons in a human brain.
Even if making faster predictions, there are some residual error compared to the actual quantum mechanical calculations. The system sometimes introduces a small noise, and sometimes it makes a wild guess if the input is very different from anything it has learned.
Thus, scientists currently using it as a surrogate model to avoid the pitfalls. In essence, computing properties of materials at a finite temperature usually involves many computation steps, the laborious and repetitive parts can be delegated to the cheap surrogate model.
Finally, the difference between the surrogate and the ground truth, which is the difference between the ANN and quantum mechanics, can be accounted for and subtracted from the final predictions.
With these methods, the analysts were thus able to reproduce few thermodynamic properties of water from quantum mechanics, including the density of ice and water, the difference in melting temperature for normal and heavy water, and the stability of various types of ice.
Also, the investigation uncovers a few physical bits of knowledge on what give ice and water systems their unconventional properties. A standout amongst the most eminent discoveries is that nuclear quantum fluctuations, which is the tendency for light elements such as hydrogen to behave more like a diffuse cloud rather than a localized particle, promote hexagonal packing of molecules inside the ice, which ultimately leads to the six-fold symmetry of snowflakes.
The study is published in the journal PNAS.