We all have wondered some or other time about how different tissues or organs might have developed in various sizes from the sphere of cells?
Well, the secret lies in the mechanics of embryonic tissues. These tissues exhibit a viscous (fluid-like) and an elastic (solid-like) behavior depending on the forces acting on them.
At EPFL, Erik Mailand, a PhD student, and Selman Sakar, an assistant professor of mechanical engineering, have decided to harness the mechanoresponsive rheology of cell clusters for engineering tissues with long-lasting complex morphologies.
Bioengineers have been studying animal tissues to engineer replicas for regenerative medicines and drug screening for a long time.
“We want to provide the cells the right mechanical cues so that their desired state coincides with our blueprint for the tissue,” says Sakar, the head of EPFL’s MicroBioRobotic Systems (MICROBS) Laboratory. “We repeatedly observed that cells are inclined to collapse the tissue into a ball due to the emergence of surface stresses.”
Therefore, to better understand the physical principles of self-organization, Sakar’s group studied the behavior of both individual cells and microfabricated tissues. Their findings have been recently published in two separate articles in Advanced Materials.
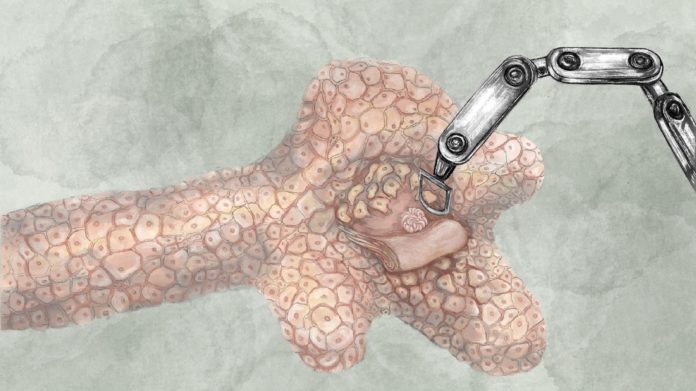
The engineers first performed robotic micromanipulation experiments to see the response of cells to forces within a fibrous matrix.
To this end, they developed a remotely controlled cell-sized magnetic microactuator that can be operated within tissues.
“This platform allows us to discover the loading conditions that would change the organization of cells. These experiments are also important to understand the onset of diseases such as fibrosis and cancer,” says Sakar.
The engineers created a one-to-one digital replica of the experimental system to quantify the mechanical stresses generated by the microactuator. “We used the digital twin to virtually test different mechanical actuation schemes and design experiments that would reveal novel insights,” says Fazil Uslu, the lead author of the first article.
Using learning from early experiments, engineers gave their attention to controlling surface stresses.
Epithelia are robust tissues that support the structure of the embryos and organs and serve as barriers against pathogens. Notably, epithelia can become elastic, plastic, and viscous by actively remodeling cell-cell junctions and modulating the distribution of local stresses.
“We used microfabrication, computational mechanics, light-sheet microscopy, and a novel robotic micromanipulation platform to show that collagen gels covered with a contiguous epithelial sheet can be freely shaped using mechanical forces,” says Mailand, the lead author of the second article. The process involves reversible solid to fluid transitions in the epithelial sheet.
It is amenable to both additive and subtractive manufacturing techniques. The engineers demonstrated the robustness and versatility of their strategy by guiding the self-assembly of a variety of molded, carved, and assembled tissues from the base material.
The study opens up new paths for research in tissue engineering. It has given new hope for the tissues developed in the lab will have proper form and function to be implanted into a patient or used for testing therapies.
The discovery may also provide a solution to the problem of tissue vascularization. As the size of the engineered tissues gets larger, the cells residing in the core no longer have access to the surrounding medium and require – as our organs do – blood vessels for perfusion.
“Our findings indicate that it could be possible to carve tunnels directly into a tissue that would eventually be stabilized by the surrounding cells to artificially create fluidic networks.” Sakar says. Showing that endothelial cells show similar mechanoresponsive characteristics as epithelial cells are the next goal of the project.
Journal Reference
- Fazil Uslu et al., Engineered Extracellular Matrices with Integrated Wireless Microactuators to Study Mechanobiology, Advanced Materials, 2021, 33, 2102641. DOI: 10.1002/adma.202102641
- Erik Mailand et al., Tissue Engineering with Mechanically Induced Solid-Fluid Transitions, Advanced Materials, 2021, 2106149. DOI: 10.1002/adma.202106149