Numerous groups of analysts are trying different things with new material mixes made of lower-cost base metals, for example, iron, copper, or aluminum. Be that as it may, so far no one has possessed the capacity to foresee whether, how, and why these catalysts react.
In order to bridge this gap and to create the basis for understanding a new generation of catalysts, scientists at the Technical University Munich have revealed a mystery of base metal compounds.
Fischer said, “What was new about our methodology that we didn’t look at existing materials, yet rather went base up and fabricated mixes made of individual copper and aluminum atoms.”
Combining two metals at the nuclear level requires no little measure of know-how and artfulness: Within a protective argon environment, the scientific experts consolidated the metal atoms which were bound to natural mixes in a test tube, to which they at that point included a solvent.
Roland Fischer, Professor for Inorganic and Metal-Organic Chemistry said, “Naturally, we hoped that the copper and aluminum atoms would separate from the organic compounds and form a cluster together. But whether they would actually do that and what the result would be was entirely unclear.”
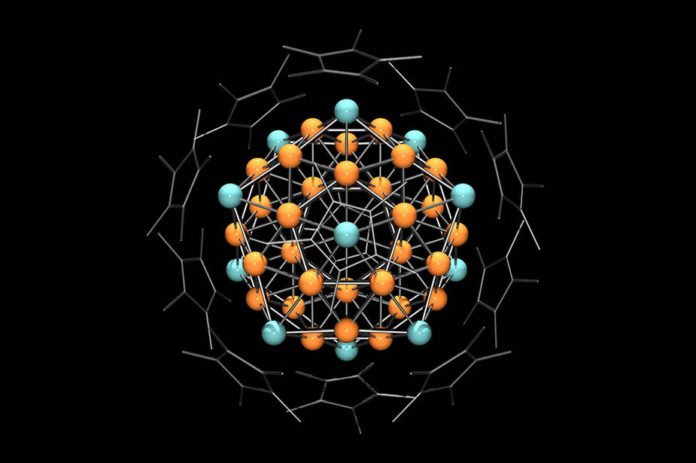
Chemists were extremely delighted to find that reddish-black particles with a diameter of up to one millimeter had formed at the bottom of the test tube. X-ray images revealed an extremely complex structure: In each case, 55 copper and aluminum atoms were arranged such that they formed a crystal whose surface consisted of 20 equilateral triangles.
Crystallographers consider such shapes icosahedrons Additional analyses demonstrated that chemically, the crystals respond like an individual copper molecule and are likewise paramagnetic, which implies that they are pulled in by an attractive field.
A clarification for the remarkable properties of the metal clusters was given by Prof. Jean-Yves Saillard from the French college in Rennes: According to him, 43 and 12 aluminum atoms sort out themselves into a “superatom” in which the metals shape a common electron shell which takes after that of a single metal molecule.
Thus, the cluster has the chemical properties of a molecule. Situated on the peripheral shell are three valence electrons whose twists adjust themselves in a magnetic field — consequently the observed paramagnetism.
Fischer said, “The heterometallic superatom by the researchers in Munich is the largest one ever made in the lab. “That it formed spontaneously, i.e. without the input of energy, out of a solution is an extremely remarkable outcome. It shows that the arrangement of 55 atoms constitutes an island of stability and hence determines the direction in which the chemical reaction takes place.”
“We are still far away from being able to use it in applications. But based on what we have now achieved, we can verify the suitability of copper-aluminum clusters for catalytic processes and also create clusters made of other promising metals.”