The cell functioning includes the interaction of proteins within the cell. The proteins dance with a partner to respond to signals and regulate each other’s activities.
Mitochondria are known as the cell’s powerhouse because it is responsible for extracting energy from food through cellular respiration. The energy is released in the form of adenosine triphosphate (ATP). It is an energy currency of the cell.
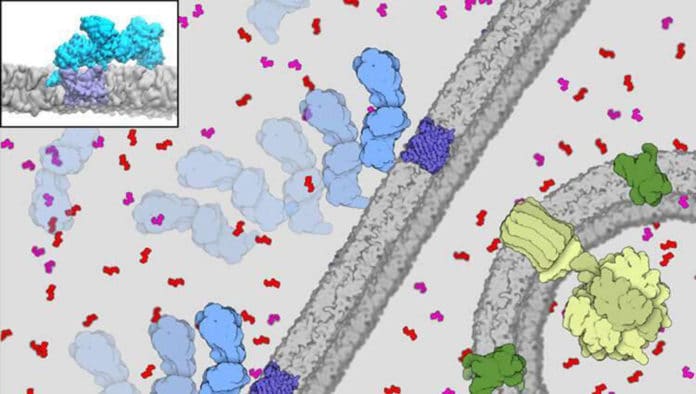
The voltage-dependent anion channel (VDAC), which is found on the outer membrane of mitochondria, binds to the protein enzyme called hexokinase-II (HKII) to charge power-hungry parts of the cells. But how VDAC binds to HKII remains obscure.
Scientists from the Texas Advanced Computing Center used supercomputer simulations to reveal how VDAC binds to HKII. The revelation could help scientists understand the molecular basis of several diseases.
The work was supported by allocations awarded by the Extreme Science and Engineering Discovery Environment (XSEDE) on the Stampede2 system of the Texas Advanced Computing Center (TACC).
Scientists found that the interaction of the enzyme with channel proteins causes the conduction of the channel to change and partially blocks the flow of ATP. Simulations on the Stampede2 system of TACC revealed this binding.
Emad Tajkhorshid, the J. Woodland Hastings Endowed Chair in Biochemistry at the University of Illinois at Urbana-Champaign, said, “If it weren’t for XSEDE, we wouldn’t be studying many of these complex projects and biological systems because you can’t afford running the simulation. They usually require long simulations, and we need multiple copies of these simulations to be scientifically convincing. Without XSEDE, it is impossible. We would have to go back to studying smaller systems.”
“A cell needs ATP to metabolize glucose. It uses the ‘P’ to convert glucose to glucose phosphate, giving it a ‘handle’ that the cell can use. Hexokinase-II makes the conversion happen, binding at the mitochondrial channel to gobble up the ATP and phosphorylate it.”
“We showed how the phosphorylation affects this process of binding between the two proteins. That was also verified experimentally.”
The VDAC channel plays a vital role in delivering ATP to hexokinase. For a healthy cell, it’s good. For a cancer cell, it also helps the cell to promote and to proliferate.
The newly developed model offers detailed and sophisticated information on the interaction of HKII and VDAC. It combines the highest-resolution all-atom molecular dynamics simulations with coarser Brownian dynamics techniques. The system size of the VDAC-HKII complex was about 700,000 atoms, including the membrane.
Po-Chao Wen, a post-doctoral research associate at the NIH Center for Macromolecular Modeling and Bioinformatics, said, “What stands out in our approach is that we considered the cellular background of this interaction.”
To study how HKII binds to the mitochondrial outer membrane, they used all-atom molecular dynamics, and a tool developed by their center called the Highly Mobile Membrane Model (HMMM), which deals with the membrane interaction.
They next used Brownian dynamics to study how HKII drifts on the membrane to meet VDAC, creating many encounter/collision events between a sitting VDAC and a drifting HKII on a planar membrane.
Wen said, “Then we used all-atom molecular dynamics to get a more refined model and specific size of the interaction to look for this particular protein-protein interaction. This helped them find the most stable complex of the two proteins formed.”
Study co-author Nandan Haloi, a Ph.D. student also at the center, said, “It seemed almost impossible when we started this process, because of the long timescales of milliseconds to seconds of the all-atom simulations.”
Tajkhorshid said, “We’re delighted with TACC and their support for not only just this work but most of our projects and also our software development, tuning the software and making it faster. TACC has been wonderful in supporting us.”
In the future, scientists are planning to include more ambitious systems such as the fusion of two cells to understand how neurons in the brain fire signals to each other.
Journal Reference:
- Nandan Haloi et al., Structural basis of complex formation between mitochondrial anion channel VDAC1 and Hexokinase-II, Communications Biology (2021). DOI: 10.1038/s42003-021-02205-y