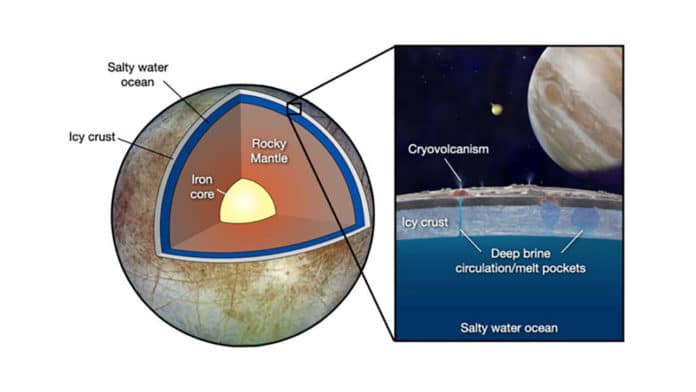
Understanding how extraterrestrial life could persist and prosper in the deep oceans of the frozen ocean worlds requires knowing the lowest temperature attainable for salty water to remain a liquid under high pressures. However, studying such worlds remains challenging because they exist at low temperatures and extremely high pressures.
In collaboration with the University of Washington, scientists at UC Berkeley have developed a new way to measure the properties of salty water with high accuracy. They can now throughput a fundamental property of aqueous solutions. It could help better understand whether the icy moons in the far reaches of our solar system can support life.
Scientists used an innovative thermodynamic technique called isochoric or constant volume to measure the lowest temperature at which salty water can remain liquid at the high pressures present in these icy moon oceans. They created an isochoric chamber that supercools aqueous solutions and self-pressurizes to prevent the formation of ice and salt-rich crystals, keeping the water liquid even at subzero temperatures.
Scientists set the desired temperature for the solution in the chamber. The system responds with the corresponding equilibrium pressure due to an often-overlooked nuance of thermodynamics in isochoric systems. This avoids time-consuming and arduous iterative testing with various temperature and pressure settings.
Among their findings, scientists discovered for the first time that despite varying concentrations and physical properties, the shift in the eutectic temperature with the application of pressure is very similar across different aqueous salt solutions. This finding could have implications for future studies and approaches to modeling icy planetary bodies.
Powell-Palm said, “This similarity further revealed that the response to the pressure of the minimum temperature for a liquid to exist is mainly controlled by the ice and is relatively insensitive to the behavior of the other solid salt phase. This provides an invaluable and fundamental rule-of-thumb for future compositional modeling of different solutes and solutions: The pressure dependence of the eutectic temperature may be roughly approximated by the melting point of pure ice.”
Collaborator Baptiste Journaux of the University of Washington said, “These findings also come at a pivotal time in space science research. Many of the icy moons in our solar system are targets for exploration over the next few decades, with missions planned by NASA’s Europa Clipper, the European Space Agency’s JUICE spacecraft, and NASA’s Dragonfly rotorcraft. The new data obtained from this study may help further researchers’ understanding of the complex geological processes observed in these icy ocean worlds.”
Journal Reference:
- Brooke Chang et al. On the pressure dependence of salty aqueous eutectics. DOI: 10.1016/j.xcrp.2022.100856