Venus flytrap is known for its unusual habit of catching and digesting insects and other small animals. There are longstanding questions about the compassionate touch response of Venus flytraps.
A new study by the scientists at Scripps Research could address those questions. The study has revealed the 3D structure of a protein channel that may enable Venus flytrap plants to snap shut.
The structure of the channel called Flycatcher1 could also offer detailed insights on how similar proteins in organisms, including plants and bacteria and proteins in the human body with similar functions (called mechanosensitive ion channels), might operate.
Mechanosensitive ion channels act as tunnels, covering the cell membranes. The channels open when they are jostled by movement, allowing charged molecules to rush. In response to this, cells change their behavior. The ability for cells to sense pressure and movement is essential for people’s senses of touch and hearing and many internal body processes.
Previous studies have highlighted three ion channels in Venus flytraps that may be related to the ability of the carnivorous plant to snap its leaves shut when its sensitive trigger hairs get touched. Flycatcher1 caught researchers’ attention because its genetic sequence looked similar to a family of mechanosensitive channels, MscS, found in bacteria.
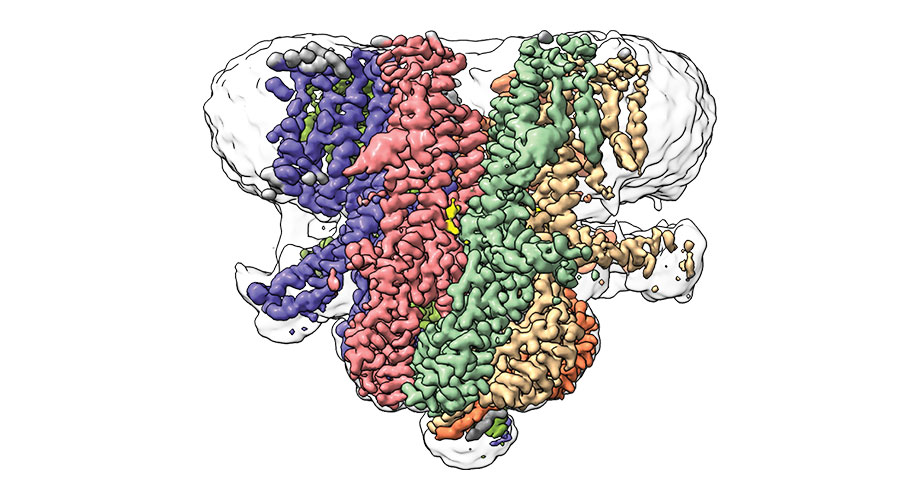
Co-first author Sebastian Jojoa-Cruz, a graduate student at Scripps Research, said, “The fact that variants of this channel are found throughout evolution tells us that it must have some fundamental, important functions that have been maintained in different types of organisms.”
In this new study, scientists used cryo-electron microscopy to identify the precise arrangement of molecules that form the Flycatcher1 protein channel in Venus flytrap plants. They found that Flycatcher1 is, in many ways, similar to bacterial MscS proteins. There is a bit of difference: Flycatcher1 has an unusual linker region extending outward from each group of helices. Like a switch, each linker can be flipped up or down.
When scientists analyzed the structure of Flycatcher1, they found six linkers in the down position, and just one flipped up.
Kei Saotome, Ph.D., a former postdoctoral research associate at Scripps Research and co-first author of the new paper, said, “The architecture of Flycatcher1’s channel core was similar to other channels that have been studied for years, but these linker regions were surprising.”
To elucidate the function of these switches, scientists altered the linker to disrupt the up position. They found that Flycatcher1 no longer functioned as usual in response to pressure; the channel remained open for a longer duration when it would normally close upon removal of pressure.
Co-senior author Swetha Murthy, Ph.D., of Vollum Institute at Oregon Health and Science University, said, “The profound effect of this mutation tells us that the conformations of these seven linkers are likely relevant for how the channel works.”
Scientists are now planning to study the function of Flycatcher1 to understand how different conformations affect its function.
Journal Reference:
- Sebastian Jojoa-Cruz, Kei Saotome, Che Chun Alex Tsui, Wen-Hsin Lee, Mark S. P. Sansom, Swetha E. Murthy, Ardem Patapoutian, Andrew B. Ward. Structural insights into the Venus flytrap mechanosensitive ion channel Flycatcher1. Nature Communications, 2022; 13 (1) DOI: 10.1038/s41467-022-28511-5
