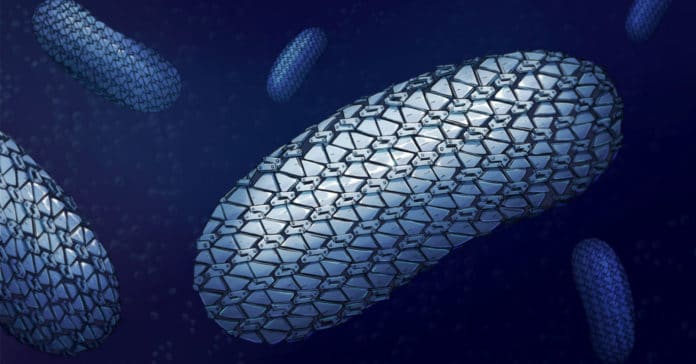
Clostridioides difficile (C. difficile) is a superbug that causes infection in large intestine. Illness from this superbug typically occurs after the use of antibiotic medications.
The bacteria protect themselves from antibiotics via a particular layer – the surface layer or S-layer. The layer covers the cell surface and plays a crucial role in cell physiology.
Using a combination of X-ray and electron crystallography, the team of scientists revealed the spectacular structure of this protective armor of superbug C.difficile.
The team includes scientists from Newcastle, Sheffield, and Glasgow Universities and colleagues from Imperial College and Diamond Light Source.
Scientists outlined the structure of the main protein, SlpA, that forms the links of the chain mail and how they are arranged to form a pattern and create this flexible armor.
They found that the SlpA crystal lattice mimics S-layer assembly in the cell through the tiling of triangular prisms above the cell wall, interlocked by distinct ridges facing the environment. The S-layer appears like the close-knit yet flexible outer layer – like chain mail.
Corresponding author Dr. Paula Salgado, Senior Lecturer in Macromolecular Crystallography who led the research at Newcastle University, said: “I started working on this structure more than ten years ago. It’s been a long, hard journey but we got some really exciting results.”
“Surprisingly, we found that the protein forming the outer layer, SlpA, packs very tightly, with very narrow openings that allow few molecules to enter the cells. S-layer from other bacteria studied so far tends to have wider gaps, allowing bigger molecules to penetrate. This may explain the success of C.diff at defending itself against the antibiotics and immune system molecules sent to attack it.”
“Excitingly, it also opens the possibility of developing drugs that target the interactions that make up the chain mail. If we break these, we can create holes that allow drugs and immune system molecules to enter the cell and kill it.”
Determining the structure of the protective armor of superbugs allows scientists to design C. diff-specific drugs to break the S-layer and create holes to allow molecules to enter and kill the cell.
Dr. Rob Fagan at the University of Sheffield said, “We’re now looking at how our findings could be used to find new ways to treat C. diff infections such as using bacteriophages to attach to and kill C. diff cells – a promising potential alternative to traditional antibiotic drugs.”
Scientists also revealed the structural and functional details of the building blocks and determined the overall X-ray crystal structure of SlpA.
Ph.D. student Paola Lanzoni-Mangutchi said, “This has been a challenging project, and we spent many hours together, culturing the difficult bug and collecting X-ray data at the Diamond Light Source synchrotron.”
Dr. Anna Barwinska-Sendra from Newcastle University said, “Working together was key to our success. It is very exciting to be part of this team and to be able to finally share our work.”
Journal Reference:
- Lanzoni-Mangutchi, P., Banerji, O., Wilson, J. et al. Structure and assembly of the S-layer in C. difficile. Nat Commun 13, 970 (2022). DOI: 10.1038/s41467-022-28196-w