Complex carbon-based particles found in meteorites have for some time been a problem for Earth-bound scientists. Thus scientists are very keen to know how exactly these particles formed from fused rings of carbon and hydrogen.
Now, scientists at UC Berkeley and Berkeley Lab have now teamed up with scientists at the University of Hawaii at Manoa and Florida International University to clarify one step in this process. This process of formation of a compound of four interlocking rings – a polycyclic aromatic hydrocarbon, or PAH – called pyrene.
PAHs are found in petroleum products on Earth and wind up as contamination when consumed, but on the other hand, they’re basic in space, particularly around Goliath, carbon-rich stars, and might be the building hinders from which life initially created. Scientists used use Berkeley Lab’s Advanced Light Source to simulate conditions around old red giant stars to create pyrene – which has 16 carbon atoms – and related PAHs.
They found that building on pyrene through even more ring expansions will produce more complex PAHs, including two-dimensional sheets of carbon like Grapheme.
To make pyrene, the specialists initially needed to make the beginning material: a three-ring, 14-carbon PAH called a 4-phenanthrenyl radical. This PAH had been orchestrated before under star-like conditions, however, scientific experts had become no further. Obviously, this PAH can’t be bought from a substance supply house.
Co-author Felix Fischer said, “These chemicals are very tedious to synthesize in the laboratory.”
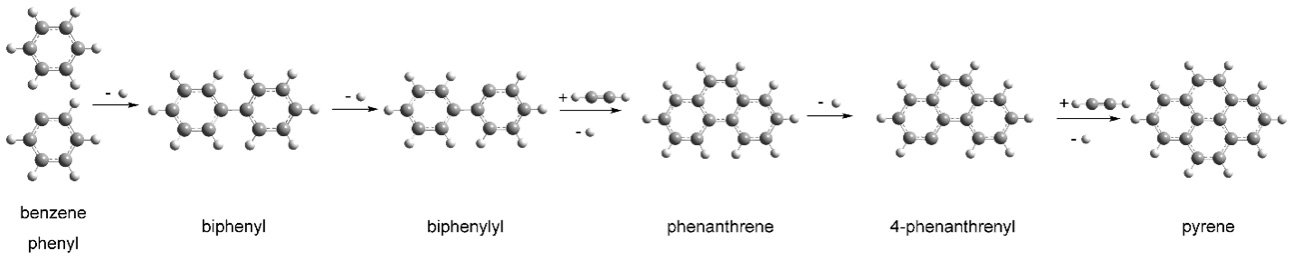
The gas mixture was then jetted out of the microreactor through a tiny nozzle at supersonic speeds, abruptly stopping the chemical reactions and allowing the scientists to analyze the intermediate reaction products.
Coupled with theoretical calculations of how the reactions should take place, the measurements confirmed the production of pyrene.
Co-author Musahid Ahmed, a scientist in Berkeley Lab’s Chemical Sciences Division said, “This is how we believe some of the first carbon-based structures evolved in the universe.”
The study is published this week in the journal Nature Astronomy.