It is difficult to image deep tissues with light. Scientists are frequently only able to observe a millimeter or two deep within a tissue due to bodily structures and chemicals’ rapid absorption and scattering of visible light. If they are successful in probing farther, chemicals like collagen or melanin frequently obstruct the image by fluorescing naturally, akin to background noise.
Using it to image deep tissue is like trying to observe the stars in daylight. The signals get flooded out. To wade out from these muddied waters, biomedical and genetic engineers at Duke University and the Albert Einstein College of Medicine have designed a small fluorescent protein that emits and absorbs light that penetrates deep into biological Tissue. The protein absorbs and emits longer wavelengths of light in the near-infrared (NIR) spectrum, allowing scientists to capture deeper, cleaner, more precise biomedical images.
Junjie Yao, assistant professor of biomedical engineering at Duke, said, “Tissue is the most transparent in the 700-1300 nanometer window of NIR light. Light can penetrate deeper into tissue at those wavelengths, and because there is less natural background fluorescence to filter out, we can take longer exposures and capture clearer images.”
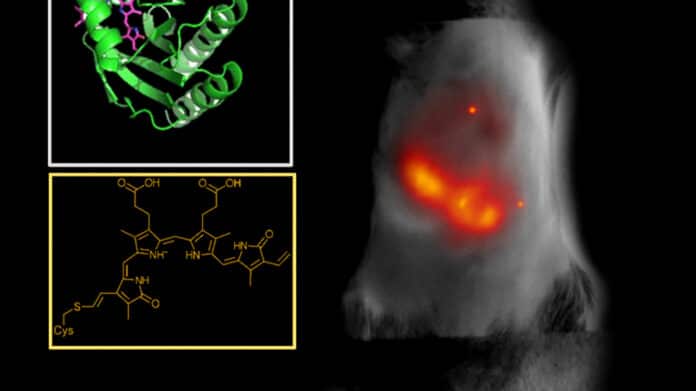
Scientists used a directed molecular evolution process to engineer their proteins, using photoreceptors normally found in bacteria as the basis for the structure. These photoreceptors can flip between an inactive and active state when exposed to a particular wavelength of light, making them suitable for the imaging study. They can bond with biliverdin, a biomolecule abundant in the tissues of mammals and people.
Vladislav Verkhusha, professor of genetics at Albert Einstein College of Medicine, New York, said, “We studied the structure of the biliverdin to determine how the photoreceptor would best attach to the biomolecule. After we understood the binding process, we carefully introduced substitutions of key parts of the molecule connecting to the biliverdin to increase the electron binding, which is necessary to obtain the red-shifted fluorescence. Last, we performed random mutagenesis followed by high-throughput screenings so the proteins would evolve and increase their brightness.”
The protein that shines the brightest, known as miRFP718nano, is easily made in cells and tissues and produces light that is just outside the visual spectrum. While the NIR activation is useful in and of itself, the subsequent period of activity offers much more promise for biological imaging.
Yao said, “We’ve seen that the NIR range can be broken into two main zones. When NIR light hits these proteins, they emit light in the first zone, about 700-900 nanometers. But as they decay, the wavelength gradually gets longer, like the tail behind a comet. That’s when they begin to emit light in the second NIR zone, which is from 900-1300 nanometers.”
All advantages of using zone one NIR light with a shorter wavelength are increased in this second zone: background fluorescence is significantly reduced, light can penetrate tissue twice as deep, and image resolution can be two to three times clearer, enabling detailed images of intricate structures.
Scientists used an imaging technique called short-wavelength infrared (SWIR) to test the efficacy of the new protein. This technique emits NIR zone-one light deep into the Tissue to activate the fluorescent proteins. Proteins produce NIR zone-two light as they degrade, which can create high-resolution images by revealing details about the structure and makeup of the targeted tissues and cells.
The scientists employed altered miRFP718nano proteins to view cells in a mouse mammary gland, take photos of bacteria in the mouse digestive system, and even follow changes to inflammation in a mouse liver after introducing them to their animal models. Compared to images created using a typical NIR zone-one imaging protein, all of the images that were recorded were crisper and more detailed.
Scientists are optimistic that their continued partnership will be a boon for their work in biomedical imaging and protein engineering. Yao is eager to use the new technique to view the brain and possibly track the migration of cancer cells as Verkhusha continues to develop and perfect fluorescent proteins and biosensors.
Journal Reference:
- Oliinyk, O.S., Ma, C., Pletnev, S. et al. Deep-tissue SWIR imaging using rationally designed small red-shifted near-infrared fluorescent protein. Nat Methods (2022). DOI: 10.1038/s41592-022-01683-0