Many hazardous diseases are caused or exacerbated by a difference in only one nucleotide building block in the all-inclusive hereditary DNA code. Such “point changes” can hand a solitary cell over the human body into a destructive cell that develops into a tumor, or transforms anti-infection touchy into anti-toxin safe microscopic organisms that reason untreatable diseases.
In a perfect world, clinicians would have the capacity to evacuate cells with such pernicious point changes directly after they were made to battle illnesses considerably more adequately.
An exploration group at the Wyss Institute for Biologically Inspired Engineering at Harvard University reports now in the Proceedings of the National Academy of Sciences (PNAS) that it has achieved the initial move toward this objective by changing over the CRISPR/Cas9 genome-designing framework into a genome-reconnaissance instrument.
The scientists led by Wyss Institute center employees George Church and James Collins, built up an in vivo transformation counteractive action strategy that empowers the DNA-cutting Cas9 protein to separate between genomic target destinations contrasting by a solitary nucleotide and cut just the undesirable one. In confirmation of-idea ponders performed in bacterial E. coli strains developed in culture or the mouse gastrointestinal tract, the approach can keep the survival of anti-microbial safe variations.
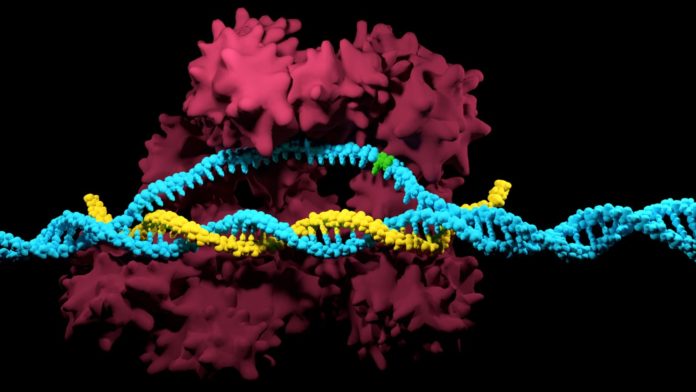
The Cas9 compound is guided to its genomic target grouping by a little guide RNA with a correlative succession. Once brought into position on the quality of intrigue, Cas9 acts like an atomic match of scissors, cutting the objective arrangement at a characterized site. In the event that repaired, this purposely acquainted harm permits researcher with altering the genome of cells, yet in the event that left unrepaired, it will make cells bite the dust.
However notwithstanding the framework’s adequacy in finding and cutting target arrangements in the genomes of numerous creatures, unspecific action that lets Cas9 haphazardly cut at optional, not totally indistinguishable destinations still represents an issue to genome engineers. This additionally implies an objective arrangement conveying an undesired point change regularly can’t be adequately separated by Cas9 from its ordinary partner and specifically evacuated. Much originator Cas9 chemicals built to be more particular so far are not ready to tackle this issue.
Church said, “By focusing instead on guide RNA features, our approach dramatically enhances Cas9’s specificity up to a level where single nucleotide polymorphisms can be clearly distinguished and unwanted genetic variants erased. Our method opens up an entirely new way to think about disease prevention in the future.”
Alejandro Chavez, a first and co-corresponding author on the study said, “We hypothesized that for a given pair of targets that differ by a single point mutation, a set of mismatches could be identified in the guide RNA that would eliminate Cas9’s activity on the normal sequence while maintaining robust activity on the one with a deleterious point mutation. This would stop a cell with a mutation cold in its tracks right after it is born.”
To build up their approach, the group utilized known point changes that happen in bacterial chemicals, furnishing pathogens with protection from anti-toxins. By concentrating on a few of these transformations and screening through guide RNA variations with various jumble blends, they could recognize particular guide RNAs that empowered Cas9 movement toward the changed quality successions, however, left the typical partner untouched.
Co-first author Benjamin Pruitt, a former staff research scientist at the Wyss Institute said, “The mutation-prevention system maintained antibiotic sensitivity not only in E. coli strains cultured in standard laboratory conditions, but also in bacteria that were used to colonize the gastrointestinal tract of gnotobiotic mice. These multiday mouse experiments involved sustained antibiotic dosing and demonstrated that the system is robust even when subjected to potentially significant environmental pressures.”
According to scientists, the strategy could more immediately be used to help the biotech industry protect large-scale cultures from acquiring mutations that render them unproductive or prone to contaminations and to study microbial evolution.
Collins, who is also the Termeer Professor of Medical Engineering & Science at MIT said, “This strategy gives us the opportunity to study evolutionary mechanisms in microorganisms. For example, we are now able to prevent often-occurring mutations that confer antibiotic resistance and ask which other genetic changes could lead to the same result. This may improve our understanding of resistance mechanisms and potentially offer new therapeutic entry points.”
Wyss Institute Director Donald Ingber said, “This collaborative effort at the Wyss Institute has resulted in an entirely new innovation in the CRISPR/Cas9 field, one that could lead to entirely new ways to prevent and treat a broad range of diseases.”
Additional authors on the study are Wyss Institute researchers Rebecca Shapiro, a former member of Collins’ group and now an assistant professor at the University of Guelph, Canada; and Marcelle Tuttle, Ryan Cecchi, Jordan Winston, Brian Turczyk, and Michael Tung, who are past and present members of Church’s group.