Human speech and language are among the most complex motor and cognitive abilities. The discovery of a mutation in the transcription factor FOXP2 in KE family members with speech disturbances has been a landmark example of the genetic control of vocal communication in humans. Cellular mechanisms underlying this control have remained unclear.
A new study from MIT and National Yang Ming Chiao Tung University sheds light on how this gene controls the ability to produce speech.
Foxp2 mutations affect the development of dendrites and neuronal synapses in the brain’s striatum, which is crucial for the regulation of movement, according to research using FOXP2 mutation/deletion mice models. The high-frequency sounds that mice use to communicate with one another were also less effective in animals with these alterations.
According to scientists, those malfunctions arise because Foxp2 mutations prevent the proper assembly of motor proteins, which move molecules within cells.
Ann Graybiel, an MIT Institute Professor, a member of MIT’s McGovern Institute for Brain Research, and an author of the paper, said, “These mice have abnormal vocalizations, and in the striatum there are many cellular abnormalities. This was an exciting finding. Who would have thought that a speech problem might come from little motors inside cells?”
In the present study, the researchers used ultrasonic vocalisations in mice as a proxy for speech to examine how the Foxp2 mutation, which has been related to apraxia, impacts speech production. These vocalisations are produced by many rodents and other creatures, including bats, to interact with one another.
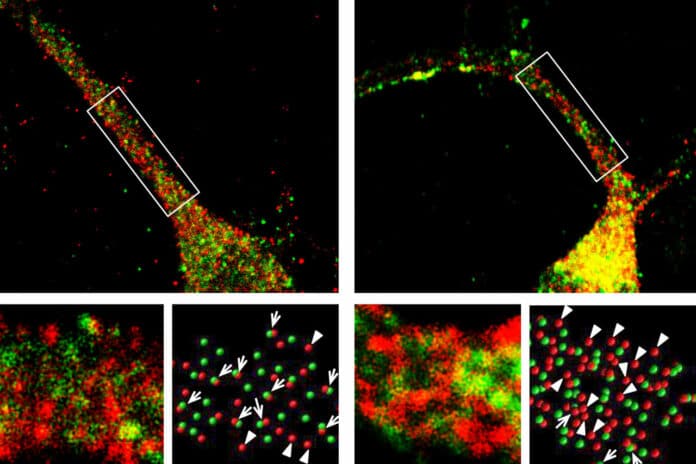
Past studies have suggested that Foxp2 affects dendrite growth and synapse formation. However, the mechanism behind this remains obscure. This study identified the mechanism through which Foxp2 affects motor proteins. The dynein protein complex, a sizable collection of proteins, is one of these molecular motors, moving molecules along microtubule scaffolds in cells.
Ann Graybiel, an MIT Institute Professor, a member of MIT’s McGovern Institute for Brain Research, and an author of the paper, said, “All kinds of molecules get shunted around to different places in our cells, and that’s certainly true of neurons. There’s an army of tiny molecules that move molecules around in the cytoplasm or put them into the membrane. In a neuron, they may send molecules from the cell body all the way down the axons.”
Several other proteins make up the dynein complex. The most significant of them is dynactin1, a protein that interacts with microtubules to allow the dynein motor to move along them. According to the results of a recent study, one of the main targets of the Foxp2 transcription factor is dynactin1.
Scientists mainly focused on the striatum, the region in which Foxp2 is frequently found. They found that the mutated version of Foxp2 is unable to suppress dynactin1 production.
Without its brake, cells produce an excessive amount of dynactin1. The delicate dynein-dynactin1 balance is disturbed. As a result, they prevent the dynein motor from moving along microtubules.
Those motors are required to move the molecules required for dendritic growth and synapse formation on dendrites. Neurons are unable to form synapses to produce the appropriate electrophysiological signals required to enable speech generation when such molecules remain stranded in the cell body.
Ultrasonic vocalisations, which generally have a frequency of between 22 and 50 kilohertz, were aberrant in mice with the Foxp2 mutation. By inhibiting the gene that codes for dynactin1, scientists demonstrated that they could reverse these vocalisation abnormalities as well as the defects in molecular motor activity, dendritic development, and electrophysiological activity.
Journal Reference
- Hsiao-Ying Kuo, Shih-Yun Chen, Rui-Chi Huang, Hiroshi Takahashi, Yen-Hui Lee, Hao-Yu Pang, Cheng-Hsi Wu, Ann M Graybiel, Fu-Chin Liu. Speech- and language-linked FOXP2 mutation targets protein motors in striatal neurons. Brain. DOI: 10.1093/brain/awad090