Usually, Lithium-ion (Li-ion) batteries are used to charge the phone, laptop, or even just a light bulb. When the battery gets fully charged, the negative electrode Li metal expands. And when it discharged, the negative electrode deflated. This makes rapid changes in size that leads to undesirable branch-like growths of Li on the surface of the Li metal. This damage builds up over time and can pose safety hazards like overheating or fire. So, to boost the energy density of a battery, scientists at the University of Maryland, have created safer batteries inspired by wood.
According to scientists, this novel design for the safer batteries could efficiently boost the energy density of a battery. Thus, it increases the power available for portable electronics and electric vehicles by reducing the risk of the battery overheating.
Research led, Liangbing Hu said, “This is part of our ongoing research to use natural materials to improve batteries. Using nature’s bio-structure, we can find inspiration to create new ways of storing energy, and we can use renewable materials, too.”
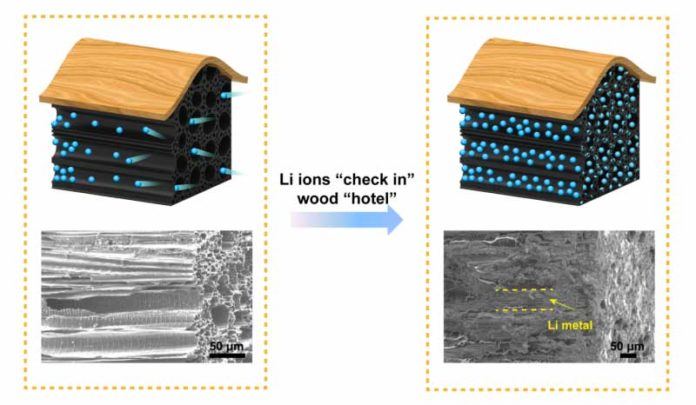
This new method for safer batteries store Li metals in the natural channels of wood. The wood channels inside it are used to carry water and nutrients. Although, the wood act as a chamber that provides huge bandwidth to store a number of Li metals. As Li metals enter in wood, it stores them comfortably and securely by maintaining the wood’s rigid exterior structure.
By doing this procedure, the battery could run safely even with fast charge and discharge rates. It has a high current density is equal to having excessive Li metals flow into/out of the wood. This may cause problems during occur at the door. And this is the main approach used in this research. Scientists just increased the number of doors available to the Li ions as they enter the wood, and avoided the pile-ups. This makes a few Li-metals passing through each gate, that’s what called local current density.
So, by using the large surface area provided by the wood, scientists minimized the local current density and created the controlled movement of Li metal.
While experiment, scientists found that the wood structure with its many straight channels, provides plenty of doors for Li-metals. This makes Li-metals to be corralled into each channel by behaving in an orderly, predictable manner even under high current density. It also avoids branch-like structure of Li that can cause battery failure.