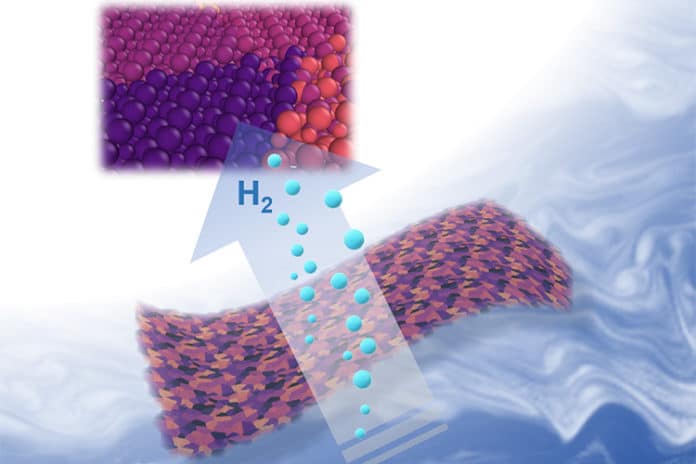
Scientists at the UC Berkeley have developed a new, efficient catalyst that can produce hydrogen fuel from water. Working as effective as Platinum, this made of Jell-O catalyst is an inexpensive new material for important catalytic reactions.
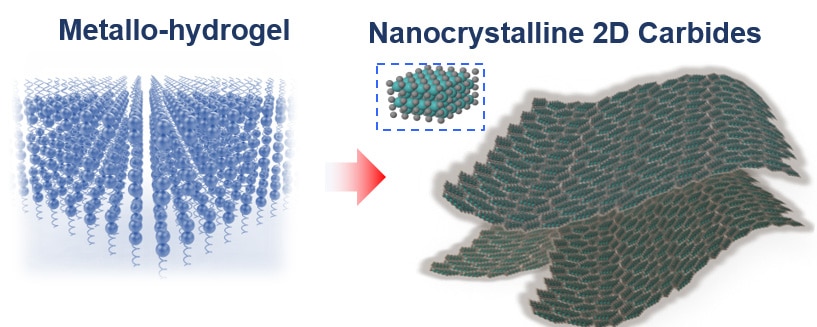
Scientists used a similar recipe of making Jell-O from a box to create this catalyst. They used nanometer-thin sheets of metal carbide and gelatin for the assembly process. They mixed gelatin and a metal ion — either molybdenum, tungsten or cobalt — with water, and then let the mixture dry.
First author Xining Zang, who conducted the research as a graduate student in mechanical engineering at UC Berkeley said, “The traditional way of using water gas to generate hydrogen still dominates in industry. However, this method produces carbon dioxide as a byproduct.”
“Electrocatalytic hydrogen generation is growing in the past decade, following the global demand to lower emissions. Developing a highly efficient and low-cost catalyst for electrohydrolysis will bring profound technical, economic and societal benefit.”
Senior author Liwei Lin, professor of mechanical engineering at UC Berkeley said, “We believe that as gelatin dries, it self-assembles layer by layer. The metal ion is carried by the gelatin, so when the gelatin self-assembles, your metal ion is also arranged into these flat layers, and these flat sheets are what give Jell-O its characteristic mirror-like surface.”
Scientists then heated the mixture at 600 degrees Celsius. Doing this makes metal ion to react with carbon atoms in the gelatin, forming large, nanometer-thin sheets of metal carbide. The unreacted gelatin burns away.
For testing, scientists placed the catalyst in water and then run an electric current through it. At the point when stacked up against one another, molybdenum carbide split water the most effectively, trailed by tungsten carbide and after that cobalt carbide, which didn’t form thin layers and add the other two. Blending molybdenum particles with a little measure of cobalt supported the execution much more.
The two-dimensional shape of the catalyst is one of the reasons why it is so successful. That is because the water has to be in contact with the surface of the catalyst in order to do its job, and the large surface area of the sheets means that the metal carbides are extremely efficient for their weight.
Lin said, “We found that the performance is very close to the best catalyst made of platinum and carbon, which is the gold standard in this area. This means that we can replace the very expensive platinum with our material, which is made in a very scalable manufacturing process.”
The work appears in the Dec. 13 print edition of the journal Advanced Materials.
Co-authors on the study are Lujie Yang, Buxuan Li and Minsong Wei of UC Berkeley, J. Nathan Hohman and Chenhui Zhu of Lawrence Berkeley National Lab; Wenshu Chen and Jiajun Gu of Shanghai Jiao Tong University; Xiaolong Zou and Jiaming Liang of the Shenzhen Institute; and Mohan Sanghasadasa of the U.S. Army RDECOM AMRDEC.