“A Second Chance for a Healthy Heart” refers to the opportunity for individuals to improve their heart health even after experiencing certain conditions or lifestyle choices that may have negatively impacted their hearts.
Heart health is crucial for overall well-being, as the heart is responsible for pumping blood and oxygen throughout the body. However, several factors, such as poor diet, lack of exercise, smoking, stress, and genetics, can contribute to heart disease.
Researchers have discovered a way to turn back the clock after a heart attack by using RNAs to instruct cells in an injured heart to eliminate scar tissue and recreate cardiac muscle, allowing the heart to function like new again. The scar tissue generated in the heart after a heart attack is tough and nonflexible and can prevent the organ from functioning at its full potential.
The study used cellular reprogramming to transform fibroblasts, a type of cell that contributes to the formation of connective tissue, into heart muscle cells. This discovery may help reverse the effects of a heart attack, the leading cause of death worldwide.
Conrad Hodgkinson, an associate professor of medicine and pathology at Duke University School of Medicine who oversaw the study, said, “We found that if you take cardiac fibroblasts from juveniles, they reprogram very nicely. But if you take cardiac fibroblasts from adults, they don’t respond at all. So, we tried to understand whether the aging process was interfering with fibroblast reprogramming.”
Conrad Hodgkinson and his team discovered that a protein oxygen sensor, Epas1, prevents adult fibroblasts from reprogramming themselves. By blocking Epas1 in adult fibroblasts, they could harness the regenerative capacity of young cells and convert more fibroblasts into cardiac muscle.
The researchers formulated a cocktail of RNAs packaged into exosomes, which they used to deliver instructions to the fibroblasts in a mouse that had just experienced a heart attack. The results were impressive, as almost all of the cardiac function lost after a heart attack was recovered.
The researchers believe this technology has limitless future applications, including restoring neuron loss in dementia patients and eliminating skin scarring in psoriasis patients without invasive surgical interventions.
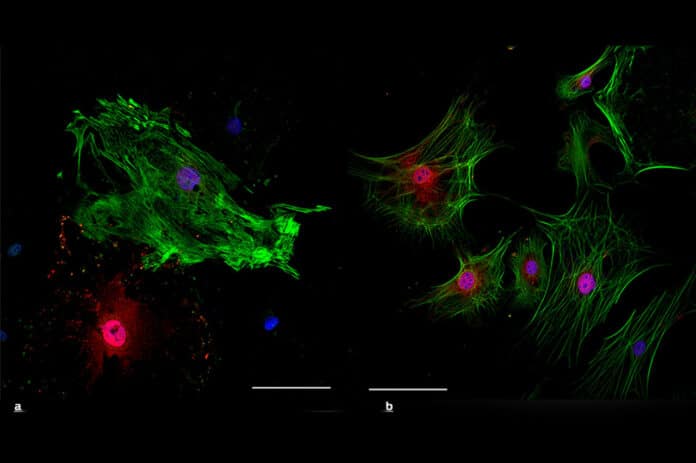
This article discusses the limitations of using miRNAs or transcription factors to reprogram fibroblasts into cardiomyocytes in the context of cardiac injury. The researchers hypothesized that manipulating the transition from neonatal to adult cardiac fibroblast phenotypes could improve the therapeutic benefits of reprogramming. They found that neonatal cardiac fibroblasts displayed a pro-cardiomyogenic phenotype, while adult cardiac fibroblasts displayed a pro-angiogenic phenotype.
The transition from the neonatal to adult phenotype depended on the transcription factor Epas1, which played a dual role in stimulating angiogenic genes while inhibiting cardiomyocyte genes. By reverting adult cardiac fibroblasts to their neonatal phenotype via Epas1 knockdown, the researchers improved reprogramming efficacy and enhanced functional improvements in infarcted adult mice.
This experiment discusses the limitations of using miRNAs or transcription factors to reprogram fibroblasts into cardiomyocytes in the context of cardiac injury. The researchers hypothesized that manipulating the transition from neonatal to adult cardiac fibroblast phenotypes could improve the therapeutic benefits of reprogramming.
They found that neonatal cardiac fibroblasts displayed a pro-cardiomyogenic phenotype, while adult cardiac fibroblasts displayed a pro-angiogenic phenotype. The transition from the neonatal to adult phenotype depended on the transcription factor Epas1, which played a dual role in stimulating angiogenic genes while inhibiting cardiomyocyte genes.
By reverting adult cardiac fibroblasts to their neonatal phenotype via Epas1 knockdown, the researchers improved reprogramming efficacy and enhanced functional improvements in infarcted adult mice.
The study found that neonatal and adult cardiac fibroblasts have distinct phenotypes, with neonatal cardiac fibroblasts displaying a pro-cardiomyogenic phenotype and adult cardiac fibroblasts expressing a pro-angiogenic phenotype. The transition from neonatal to adult phenotype was found to be dependent on the transcription factor Epas1. Epas1 played a dual role in stimulating angiogenic genes while inhibiting cardiomyocyte genes.
Reverting adult cardiac fibroblasts to their neonatal phenotype via Epas1 knockdown improved reprogramming efficacy in adult mice, leading to enhanced functional improvements associated with reprogramming in infarcted adult mice.
These findings suggest that manipulating the mechanism whereby fibroblasts transition from their neonatal to adult phenotype may enhance the therapeutic benefits of cardiac reprogramming.
Journal Reference:
- Hualing Sun,Richard E. Pratt,etal. Neonatal and adult cardiac fibroblasts exhibit inherent differences in cardiac regenerative capacity. Journal of Biological Chemistry DOI:10.1016/j.jbc.2023.104694