Mercury can seep into water supplies from improperly discarded devices containing it, as runoff from landfills & farmland, dumped by factories, or from natural deposits. It is extremely toxic and precautions must be taken when handling or disposing of it, as mercury can be absorbed through the skin and inhaled.
Though, boiling your water will not remove mercury from it. Now, researchers from Chalmers University of Technology, Sweden, present a totally new way to filter out mercury-contaminated water, through an electrochemical process.
In the last two years, Björn Wickman and Cristian Tunsu, a researcher at the Department of Chemistry and Chemical Engineering at Chalmers, have studied an electrochemical process for cleaning mercury from water. Their method works via extracting the heavy metal ions from water by encouraging them to form an alloy with another metal.
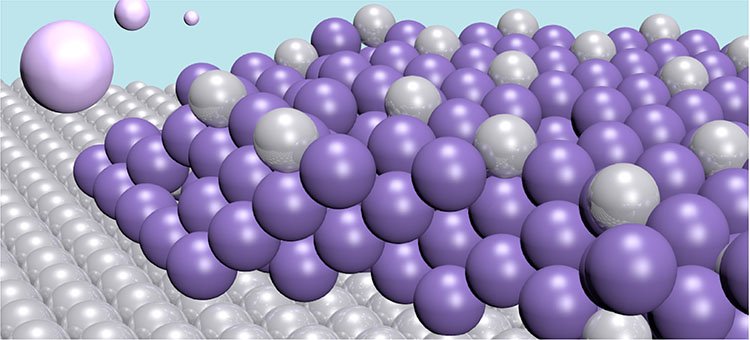
The method includes an electrode plate that binds specific heavy metals to it. The electrode is made of the noble metal platinum, and through an electrochemical process, it draws the toxic mercury out of the water to form an alloy of the two. In this way, the water is cleaned of the mercury contamination. The alloy formed by the two metals is very stable, so there is no risk of the mercury re-entering the water.
The electrode used in this technique has a very high capacity. Each atom of the platinum can bond with four mercury atoms. Furthermore, the mercury atoms do not only bond on the surface but also penetrate deeper into the material, creating thick layers. This means the electrode can be used for a long time.
And the fascinating is, the electrode can be recycled, and the mercury disposed of in a safe way. A further positive for this process is that it is very energy efficient.
Björn Wickman said, “Another great thing with our technique is that it is very selective. Even though there may be many different types of substance in the water, it just removes the mercury. Therefore, the electrode doesn’t waste capacity by unnecessarily taking away other substances from the water.”
Scientists have published their study in the journal Nature Communications.
