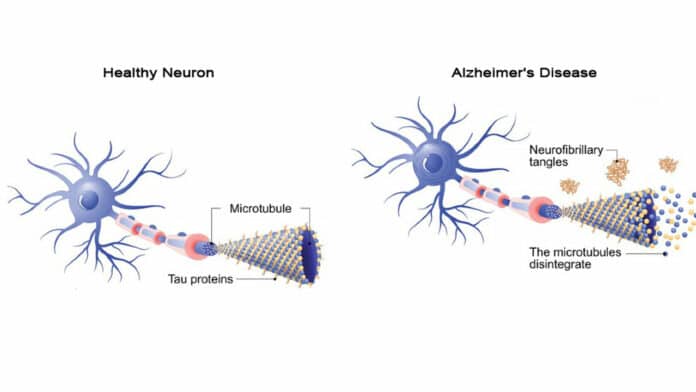
The six subtypes of tau, which are comprised of three subtypes grouped as 4R-tau and three subtypes together as 3R-tau, are present at almost equal levels in normal human brain cells. According to scientists, when there is a change in the ratio of 4R-tau subtypes, this rise has adverse effects that lead to early cognitive impairment. The exact impact is also shown in older animals.
To test this ratio shift theory, researchers from Bristol‘s Schools of Physiology, Pharmacology, Neuroscience, and Biochemistry examined the 4R-tau subtypes in more detail and the acute effects of shifting the balance of the different subtypes on brain cell nerve activity.
The study is the first to investigate how changing the balance of a crucial protein called tau may affect neuronal function. Alzheimer’s disease and dementia are characterized by increased protein levels, which build up and create tangles in the brain. Tau’s role in early cognitive decline has not yet been fully explored, although it is well known to be one of the vital harmful processes underlying these disorders.
In animal models, the researchers discovered that two 4R-tau subtypes enhanced L-type calcium channel density in brain cells, whereas the third subtype did not. The other two 4R-tau subtypes’ effects were brought about by tau’s direct protein-protein interaction with the calcium channel. The slow afterhyperpolarization, an improved inhibitory response, decreased the brain cell activity due to this increase in L-type calcium channels (which also happens in old animals) (slow AHP). The L-type calcium channel, a protein ion channel, is where calcium ions enter the brain cell to cause the sluggish AHP.
The team showed that the effect in the hippocampus resulted from more calcium entering the neurons through L-type calcium channels, which supports existing evidence that nerve death in dementia is caused by calcium overload.
Their discovery shows that changes that occur in a hippocampal nerve during early cognitive impairment are changes that occur before the patient is finally diagnosed with dementia.
Professor Neil Marrion, Professor of Neuroscience at Bristol’s School of Physiology, Pharmacology & Neuroscience, and the study’s lead author said: “Our exciting findings suggest that by preventing the direct interaction between 4R-tau subtypes and L-type calcium channels could be used as a therapeutic approach to prevent the onset of dementia.”
Journal Reference:
- Stan, G.F., Church, T.W., Randall, E., et al. Tau isoform-specific enhancement of L-type calcium current and augmentation of afterhyperpolarization in rat hippocampal neurons. Sci Rep 12, 15231 (2022). DOI: 10.1038/s41598-022-18648-0