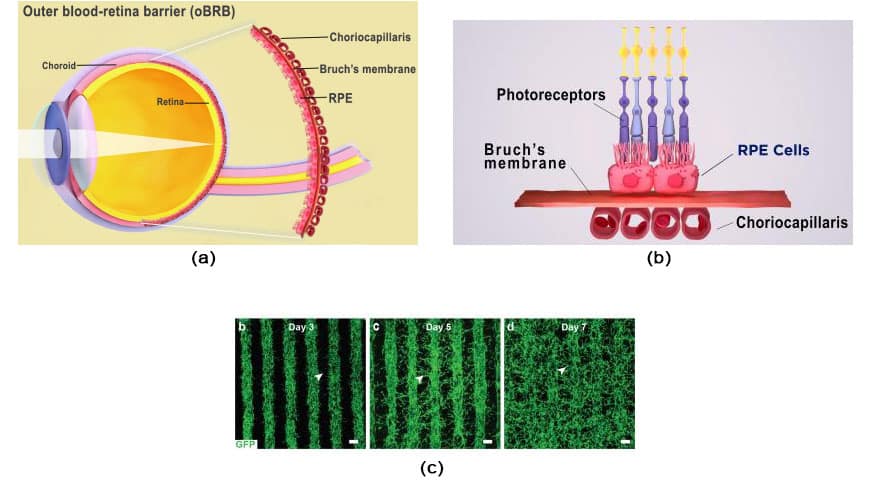
Age-related macular degeneration (AMD) is a leading cause of blindness. It initiates in the outer-blood-retina-barrier (oBRB) formed by the retinal pigment epithelium (RPE), Bruch’s membrane, and choriocapillaris. AMD initiation and progression mechanisms still need to be better understood owing to the lack of physiologically relevant human oBRB models.
The National Eye Institute (NEI) research team, part of the National Institutes of Health, used patient stem cells and 3D bioprinting to produce eye tissue that will advance the understanding of the mechanisms of blinding diseases. Scientists printed a combination of cells that form the outer blood-retina barrier.
The retinal pigment epithelium (RPE), separated from the blood vessel-rich choriocapillaris by Bruch’s membrane, makes up the outer blood-retina barrier. The choriocapillaris and RPE exchange nutrients and waste under the control of Bruch’s membrane. Drusen, which are lipoprotein accumulations, develop outside of the Bruch’s membrane in AMD and obstruct its function. RPE degradation over time causes photoreceptor deterioration and vision loss.
Scientists combined three immature choroidal cell types in a hydrogel: pericytes, endothelial cells, and fibroblasts. They then printed the gel on a biodegradable scaffold. Within days, the cells began to mature into a dense capillary network.
On day nine, the scientists seeded retinal pigment epithelial cells on the flip side of the scaffold. The printed tissue reached full maturity on day 42. Tissue analyses and genetic and functional testing showed that the printed tissue looked and behaved similarly to native outer blood-retina barrier.
When subjected to stress, printed tissue displayed early-stage AMD characteristics, such as drusen deposits under the RPE, and progressed to late-stage dry-stage AMD, where tissue breakdown was seen. Low oxygen levels caused a wet AMD-like look with choroidal vascular hyperproliferation that moved into the sub-RPE zone. When used to treat AMD, anti-VEGF medications slowed the formation and migration of blood vessels while also improving tissue shape.
Kapil Bharti, Ph.D., who heads the NEI Section on Ocular and Stem Cell Translational Research said, “By printing cells, we’re facilitating the exchange of cellular cues necessary for normal outer blood-retina barrier anatomy. For example, the presence of RPE cells induces gene expression changes in fibroblasts that contribute to the formation of Bruch’s membrane — something that was suggested many years ago but wasn’t proven until our model.”
Scientists addressed two technological issues: creating an appropriate biodegradable scaffold and achieving a consistent printing pattern. They developed a temperature-sensitive hydrogel that produced distinct rows while the gel was cold but dissolved when the gel warmed. A more exact system of assessing tissue architecture was made possible by good row consistency. Additionally, they optimized the proportion of fibroblasts, endothelial cells, and pericytes in the cell combination.
Co-author Marc Ferrer, Ph.D., director of the 3D Tissue Bioprinting Laboratory at NIH’s National Center for Advancing Translational Sciences, and his team provided expertise for the biofabrication of the outer blood-retina barrier tissues “in-a-well,” along with analytical measurements to enable drug screening.
“Our collaborative efforts have resulted in very relevant retina tissue models of degenerative eye diseases,” Ferrer said. “Such tissue models have many potential uses in translational applications, including therapeutics development.”
Journal Reference:
- Min Jae Song, Russ Quinn et al. Bioprinted 3D outer retina barrier uncovers RPE-dependent choroidal phenotype in advanced macular degeneration. Nature Methods, 2022; DOI: 10.1038/s41592-022-01701-1