The rapid development of the COVID-19 pandemic highlights shortcomings in the existing laboratory-based testing paradigm for viral diagnostics. The fundamental limitations of current diagnostic assays for viral pathogens stem from their reliance upon PCR analysis, which requires temperature cycling and labor-intensive, laboratory-based protocols for viral isolation, lysis, and removal of inhibiting materials.
In a new study, the University of Illinois, Urbana-Champaign researchers have demonstrated a rapid COVID-19 molecular test prototype. They also developed a portable instrument for reading the results with a smartphone in 30 minutes.
Typical tests for SARS-CoV-2 take a sample from a patient with a long nasopharyngeal swab, put that swab into a substance called viral transport media, and send it to a lab multistep process of extracting, isolating, and multiplying the telltale RNA inside the virus. This RNA multiplication process, called RT-PCR, requires several temperature fluctuation cycles, specialized equipment, and trained personnel.

Unlike that, this new test used a more straightforward process to analyze the viral transport media, called LAMP, which bypasses the RNA extraction and purification steps.
A graduate student Anurup Ganguli, the first author of the study, said, “LAMP only needs one temperature – 65 C – so it is much easier to control. Also, LAMP works more robustly than PCR, especially when there are contaminants in the test sample. We can briefly heat the sample, break open the virus, and detect the genetic sequence that specifically identifies SARS-CoV-2.”
Scientists compared the LAMP assay with PCR, first using synthetic nasal fluid spiked with the virus and then with clinical samples. They found the results were in agreement with PCR results, and they documented the sensitivity and specificity of the LAMP test.
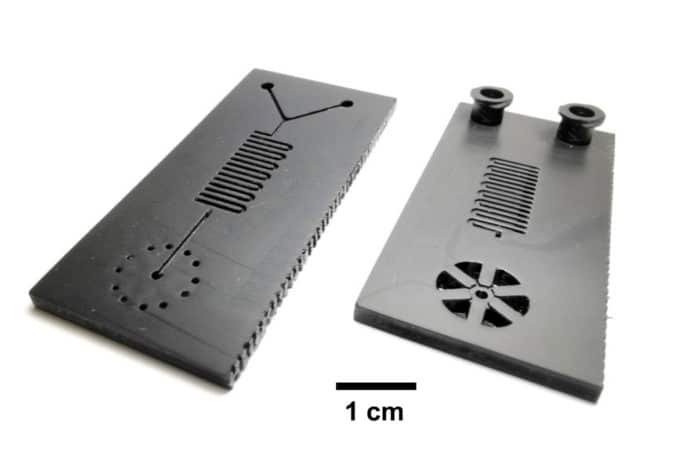
They then incorporated the LAMP assay onto a small 3D-printed microfluidic cartridge with two input slots for syringes: one for the sample-containing viral transport media, one for the LAMP chemicals. Once the two are injected, they react within the cartridge.
Mechanical science and engineering professor Bill King said, “We use modern, high-speed additive manufacturing to make these cartridges. The entire thing can be quickly scaled up to hundreds of thousands of tests. Production scale-up is typically the biggest obstacle for commercial applications of microfluidic cartridges, and we can overcome that obstacle using this new approach. Modern additive manufacturing is elastic and scalable, and it can be ramped up very quickly compared with legacy manufacturing technologies.”
The cartridge can be embedded into a hand-held portable instrument with a heating chamber, which heats the cartridge to 65 degrees Celsius for the reaction’s duration and smartphone support for reading the outcomes. In around 30 minutes, a positive outcome will emit a bright light.
Electrical and computer engineering professor Brian Cunningham said, “The reader illuminates the liquid compartments with light from blue LEDs, while the phone’s rear-facing camera records a movie of the green fluorescent light being generated.”
Bashir said, “Scientists are exploring whether the assay would work with saliva samples to eliminate the need for nasopharyngeal swabs and collecting more patient data as they consider the next steps for regulatory approvals.”
Journal Reference:
- Anurup Ganguli et al. Rapid isothermal amplification and portable detection system for SARS-CoV-2. DOI: 10.1073/pnas.2014739117