Caffeine holds the potential help people stay alert. Now, MIT scientists used the potential for a chemical stimulant in order to catalyze the formation of polymer synthesis.
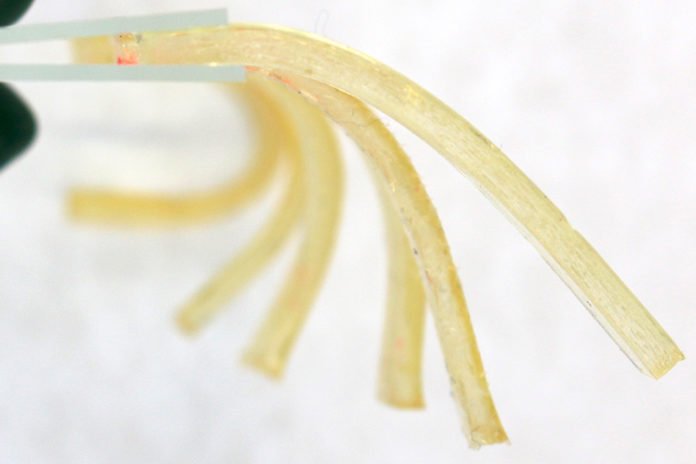
For experiments, scientists have demonstrated gummy, biocompatible gels that could be used for drug delivery and other medical applications. They expecting that the gel could be used to carry other types of drugs.
Drugs carried by this kind of material could be really appealing for patients, especially children, who have difficulty with swallowing capsules and tablets. And this new way to make gels using catalysts and starting materials that are based on food products and other materials that are safe to ingest.
Robert Langer, the David H. Koch Institute Professor at MIT said, “Most synthetic approaches for synthesizing and cross-linking polymeric gels and other materials use catalysts or conditions that can damage sensitive substances such as biologic drugs. In contrast, here we used green chemistry and common food ingredients. We believe these new materials could be useful in creating new medical devices and drug delivery systems.”
The paper appears the journal Biomaterials. Former MIT postdoc Angela DiCiccio, who is now at Verily Life Sciences, the life sciences division of Google X, is the lead author of the paper. Other MIT authors of the paper include Young-Ah Lucy Lee, Dean Glettig, Elizabeth Walton, Eva de La Serna, Veronica Montgomery, and Tyler Grant.
Scientists actually wanted to simplify the method of manufacturing and impart an improved safety profile from the beginning by using potentially safer catalysts. Caffeine acts as a weak base, thus, it can gently remove protons from other molecules.
DiCiccio said, “Polyesters allow for the intentional design of ingestible materials made from bioderived resources. However, there didn’t exist any catalysts that were mild enough to enchain these molecules without causing unwanted reactions or requiring super high heat. Our new platform provides an elegant solution to this problem using inexpensive materials and broadly accessible chemistries.”
Scientists induced caffeine with citric acid to make a polymer network along with polyethylene glycol (PEG), a biocompatible polymer that has been used in drugs and consumer products such as toothpaste for many decades. Next, they heated it, that makes caffeine opens up an oxygen-containing ring in the PEG.
The reaction also allows caffeine to react with citric acid to form chains that consist of alternating molecules of PEG and citric acid. If drug molecules are present in the mixture, they also become incorporated into the chains.
The gels can likewise be engraved with examples, for example, the microscale engineering found on the surface of lotus leaves, which enables them to repulse water. Changing the surface attributes of the material could enable specialists to control how rapidly or gradually the gels travel through the stomach related tract.
The subsequent gels contain a little measure of caffeine, generally the same as that found in some tea. In preparatory security tests, the scientists found no unsafe impacts in four sorts of human cells, or in rats.