Hydrogen is the second smallest of all the atoms available. It has the property to penetrate directly into the crystal structure of the solid metals.
This property of hydrogen is very good for storing the hydrogen fuel safely within that metal itself. But it can be worst for some other structures where the more consumption of hydrogen fuel can cause the metal walls to get more brittle and leads to failure.
As hydrogen atoms diffuse very fast even within the solid metals, this embrittlement process is difficult to detect.
To overcome this problem of detecting embrittlement, researchers at MIT have found out a way. They create a new technique which helps them observe the metal surface amid hydrogen penetration. The new way of observing the process of embrittlement from hydrogen as it happens will help find out when this embrittlement gets triggered. It will also suggest the way that how to slow down this process or avoiding it by developing a new alloy that causes less embrittlement.
Their research is described in a paper appearing in the MIT postdoc Jinwoo Kim and Thomas B. King Assistant Professor of Metallurgy C. Cem Tasan.
“It’s definitely a cool tool,” says Chris San Marchi, a distinguished member of the technical staff at Sandia National Laboratories, who was not involved in this work. “This new imaging platform has the potential to address some interesting questions about hydrogen transport and trapping in materials, and potentially about the role of crystallography and microstructural constituents on the embrittlement process.”
It could be a cheaper, lighter and safer process to store the fuel in the crystal lattice of the metal itself. But firstly, we need to understand how hydrogen atoms enter and leave the metals.
Tesan says,” Hydrogen can diffuse at relatively high rates in the metal because it’s so small. If you take metal and put it in a hydrogen-rich environment, it will uptake the hydrogen, and this causes hydrogen embrittlement.” This is because the hydrogen tends to segregate in a certain part of the metal crystal lattice, which weakens its chemical bond.
Sandia’s San Marchi says that “this method may play an important role — in coordination with other techniques and simulation — to illuminate the hydrogen-defect interactions that lead to hydrogen embrittlement. With a more comprehensive understanding of the mechanisms of hydrogen embrittlement, materials and microstructures can be designed to improve their performance under extreme hydrogen environments.”
Courtesy of the researchers
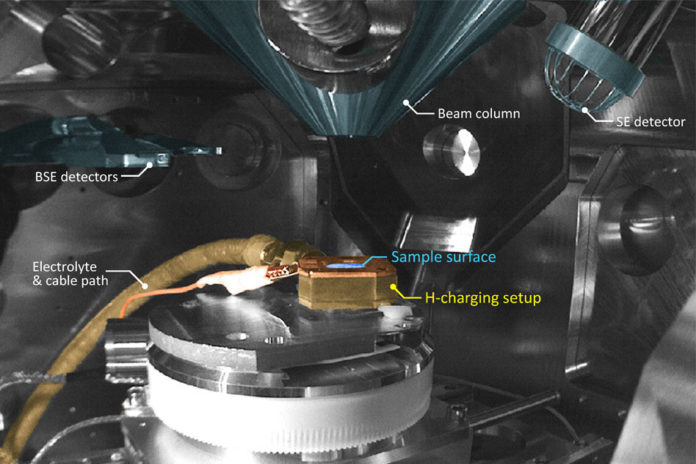
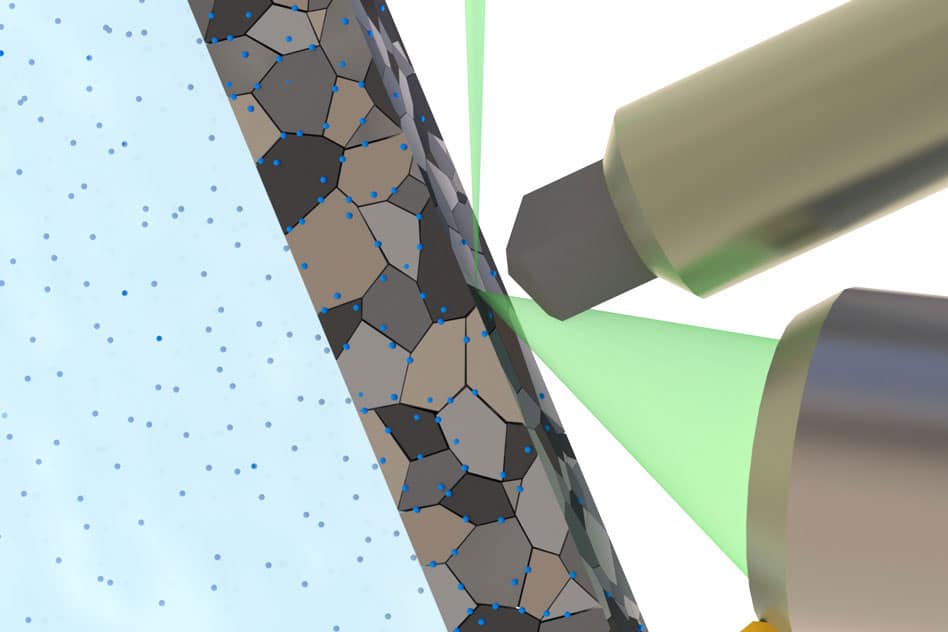
The researchers used an electrolyte that can be placed in the well-sealed chamber, where it is exposed to the lower side of the thin sheet of metals. Whereas the top of the metal is exposed to the SEM (scanning Electrode Microscope) electron beam, which can then examine the structure of the metal. Also, it monitors the effect of hydrogen atoms entering it.
Initially, they test three different metal- two different kinds of stainless steel and a titanium alloy. They observed the formation and growth of a nanophase hydride phase in the titanium alloy in real time and at the room temperature.
The electrolyte needed to charge the metal with hydrogen, “is a bit dangerous for the microscope,” Tasan says. “If the sample fails and the electrolyte is released into the microscope chamber,” it could penetrate far into every nook and cranny of the device and be difficult to clean out. When the time came to carry out their first experiment in the specialized and expensive equipment, he says, “we were excited, but also really nervous. It was unlikely that failure was going to take place, but there’s always that fear.”
Kaneaki Tsuzaki, a distinguished professor of chemical engineering at Kyushu University in Japan, who was not involved in this research, says this “could be a key technique to solve how hydrogen affects dislocation motion. It is very challenging because an acid solution for hydrogen cathodic charging is circulating into an SEM chamber. It is one of the most dangerous measurements for the machine. If the circulation joints leak, a very expensive scanning electron microscope (SEM) would be broken due to the acid solution. Very careful design and a very high-skill setup are necessary for making this measurement equipment.”
Tsuzaki adds that “once it is accomplished, outputs by this method would be super. It has a very high spatial resolution due to SEM; it gives in-situ observations under a well-controlled hydrogen atmosphere.” As a result, he says, he believes that Tasan and Kim “will obtain new findings of hydrogen-assisted dislocation motion by this new method, solve the mechanism of hydrogen-induced mechanical degradation, and develop new hydrogen-resistant materials.”