How do growing bacterial colonies get their shapes? While colony morphogenesis is well studied in two dimensions, many bacteria grow as large colonies in three-dimensional (3D) environments. However, little is known about the colony morphologies of bacteria growing in three dimensions.
Now, a Princeton team has invented a way to observe bacteria in 3-D environments. They discovered that as the bacteria multiply, their colonies consistently take on rough shapes that are significantly more intricate than those generally observed in flat dishes. These shapes resemble a branching head of broccoli.
Sujit Datta, an assistant professor of chemical and biological engineering at Princeton and the study’s senior author, said, “Ever since bacteria were discovered over 300 years ago, most lab research has studied them in test tubes or on Petri dishes. If you try to watch bacteria grow in tissues or soils, those are opaque, and you can’t see what the colony is doing. That has been the challenge.”
Datta’s research team uncovered this behavior using a breakthrough experimental setup that allowed them to make unprecedented observations of bacterial colonies in their natural, three-dimensional state. Unexpectedly, the scientists discovered that the growth of the wild colonies constantly resembled the formation of crystals or the spread of frost on a windowpane. These rough, branchy structures are common throughout nature, but they are usually seen in the context of expanding or converging nonliving systems.
Datta said, “We found that growing in 3-D, bacterial colonies exhibit a very similar process despite the fact that these are collectives of living organisms.”
Datta said, “At a fundamental level, we’re excited that this work reveals surprising connections between the development of form and function in biological systems and studies of inanimate growth processes in materials science and statistical physics. But also, we think this new view of when and where cells are growing in 3-D will interest anyone interested in bacterial growth, such as in environmental, industrial, and biomedical applications.”
For several years, Datta’s research group has been working on a system to study events typically hidden in obscure environments, including fluid flowing through soils. The team supports bacterial growth in 3-D by using specially engineered hydrogels and water-absorbent polymers similar to jello and contact lenses. Unlike those common versions of hydrogels, Datta’s materials are made up of tiny balls of hydrogel easily deformed by the bacteria, allowing for the free passage of oxygen, and nutrients that support bacterial growth are transparent to light.
Datta said, “It’s like a ball pit where each ball is an individual hydrogel. They’re microscopic, so you can’t see them. The research team calibrated the hydrogel’s makeup to mimic the structure of soil or tissue. The hydrogel is strong enough to support the growing bacterial colony without presenting enough resistance to constrain the growth.”
“As the bacterial colonies grow in the hydrogel matrix, they can easily rearrange the balls around them, so they are not trapped. It’s like plunging your arm into the ball pit. If you drag it through, the balls rearrange around your arm.”
To study how bacteria grow in three dimensions, the researchers conducted trials with four distinct types of bacteria, including one that contributes to the acidic flavor of kombucha.
Datta said, “We changed cell types, nutrient conditions, and hydrogel properties. We systematically changed all those parameters, but this appears to be a generic phenomenon.”
“Two factors seemed to cause the broccoli-shaped growth on a colony’s surface. First, bacteria with access to high levels of nutrients or oxygen will grow and reproduce faster than in a less abundant environment. Even the most uniform environments have some uneven density of nutrients, and these variations cause spots in the colony’s surface to surge ahead or fall behind. Repeated in three dimensions, this causes the bacterial colony to form bumps and nodules as some subgroups of bacteria grow more quickly than their neighbors.”
“Second, the researchers observed that only the bacteria close to the colony’s surface grew and divided in three-dimensional growth. The bacteria crammed into the colony’s center seemed to lapse into a dormant state. Because the bacteria inside were not growing and dividing, the outer surface was not subjected to pressure that would cause it to expand evenly. Instead, its expansion is primarily driven by growth along the very edge of the colony. And the growth along the edge is subject to nutrient variations that eventually result in bumpy, uneven growth.”
Alejandro Martinez-Calvo, a postdoctoral researcher at Princeton and the paper’s first author, said, “If the growth was uniform, and there was no difference between the bacteria inside the colony and those on the periphery, it would be like filling a balloon. The pressure from the inside would fill in any perturbations on the periphery.”
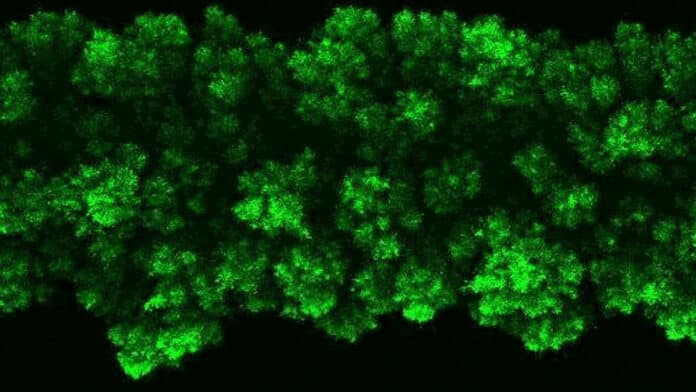
To explain why this pressure was not present, the researchers added a fluorescent tag to proteins that become active in cells when the bacteria grow. The fluorescent protein lights up when bacteria are active and remains dark when they are not. Observing the colonies, the researchers saw that bacteria on the colony’s edge were bright green, while the core remained dark.
Datta said, “The colony essentially self-organizes into a core and a shell that behave in very different ways.”
“The theory is that the bacteria on the colony’s edges scoop up most of the nutrients and oxygen, leaving little for the inside bacteria.”
“We think they are going dormant because they are starved, although he cautioned that further research was needed to explore this.”
“The experiments and mathematical models used by the researchers found an upper limit to the bumps that formed on the colony surfaces. The bumpy surface results from random variations in the oxygen and nutrients in the environment, but the randomness tends to even out in certain limits.”
“The roughness has an upper limit of how large it can grow — the floret size if we compare it to broccoli. We were able to predict that from the math, and it seems to be an inevitable feature of large colonies growing in 3-D.”
“Because the bacterial growth tended to follow a similar pattern as crystal growth and other well-studied phenomena of inanimate materials, the researchers were able to adapt standard mathematical models to reflect the bacterial growth. He said future research will likely focus on better understanding the mechanisms behind the growth, the implications of rough growth shapes for colony functioning, and applying these lessons to other areas of interest.”
“Ultimately, this work gives us more tools to understand, and eventually control, how bacteria grow in nature.”
Journal Reference:
- Alejandro Martínez-Calvo, Morphological instability and roughening of growing 3D bacterial colonies. Proceedings of the National Academy of Sciences. DOI: 10.1073/pnas.2208019119