Even after mixing oil and water together, they tend to remains separate. The oil always floats to the top because it is less dense than water. Oil and water don’t mix because water molecules are more attracted to each other than to oil molecules.
Now, MIT scientists have found a way to get the two substances to mix and stay stable for long periods no shaking required.
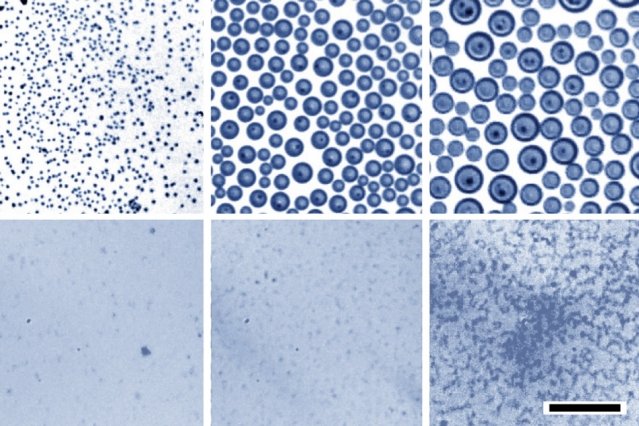
Scientists primarily cooled down the bath of oil containing a small amount of surfactants. They then let the water vapor from the surrounding air condense onto the oil surface. This causes tiny, uniform water droplets on the surface that then sink into the oil, and their size can be controlled by adjusting the proportion of surfactants.
Courtesy of the researchers
For various applications including new drug-delivery systems and food-processing methods, it is essential to get oil in water and form tiny droplets. On an industrial scale, these emulsions are made by either mechanically shaking the blend or utilizing sound waves to set up serious vibrations inside the fluid. This process is also called as sonicating.
MIT graduate student Ingrid Guha said, “The process requires a lot of energy. The finer the drops, the more energy it takes. In contrast, our approach is very energy inexpensive.”
“The key to overcoming that separation is to have really small, nanoscale droplets. When the drops are small, gravity can’t overcome them,” and they can remain suspended indefinitely.”
For this new process, scientists set up a reservoir of oil with an added surfactant that can bind to both oil and water molecules. They placed this inside a chamber with very humid air and then cooled the oil. Like a glass of cold water on a hot summer day, the colder surface causes the water vapor to precipitate. The condensing water then forms droplets at the surface that spread through the oil-surfactant mixture, and the sizes of these droplets are quite uniform.
Scientist thus produced nanoscale emulsions that remained stable over periods of several months.
Guha said, “The droplets stay so small that they’re hard to see even under a microscope. By cloaking the freshly condensed nanoscale water droplets with oil, we are taking advantage of the inherent nature of phase-change and spreading phenomena.”
Scientists believe that their work provides a kind of design guideline for someone to use for a particular kind of application.
Associate professor Kripa Varanasi said, “It’s such an important thing, because foods and pharmaceuticals always have an expiration date, and often that has to do with the instability of the emulsions in them. The experiments used a particular surfactant that is widely used, but many other varieties are available, including some that are approved for food-grade products.”
Guha said, “we envision that you could use multiple liquids and make much more complex emulsions. And besides being used in food, cosmetics, and drugs, the method could have other applications, such as in the oil and gas industry, where fluids such as the drilling ‘muds’ sent down wells are also emulsions.”
