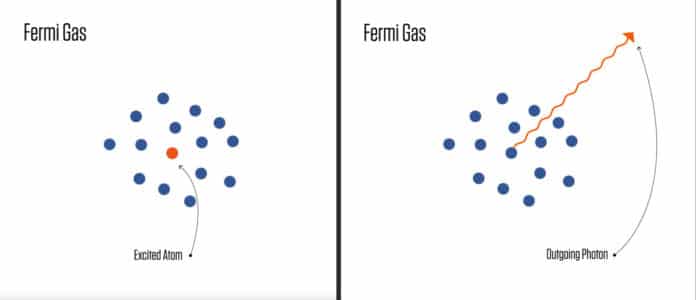
JILA scientists have created a congested ‘Fermi Sea’ that keeps atoms in a high-energy state or excited by tuning a dense quantum gas of atoms. Because of the delay in their normal return to the lowest-energy state, the atoms remain excited about 10% longer than usual.
According to scientists, this technique can be used to improve quantum communication networks and atomic clocks.
Atoms that remain excited for longer naturally calm down by releasing light in quantized portions called photons. This process is noticeable in the glow of fireflies and emission from LEDs. The rate of decay can be engineered by modifying the environment or the internal properties of the atoms.
This newly developed method is based on the Pauli exclusion principle. The principle suggests that identical fermions (a category of particles) can’t share the same quantum states simultaneously.
If there are enough fermions in the crowd, it creates a Fermi Sea, an exciting fermion that might not be able to fling out a photon as usual because it would need to recoil.
This recoil could land it to the same quantum state of motion as others. But, the Pauli exclusion principle forbids it and blocks it.
Also, the Fermi sea was large enough that atoms in the middle couldn’t escape. Atoms on the surface can’t be blocked as easily.
NIST/JILA Fellow Jun Ye said, “Pauli blocking uses well-organized quantum emotional states of a Fermi sea to block the recoil of an atom that wants to decay, thus prohibiting spontaneous decay. It is a profound quantum effect for the control of matter’s properties that were previously deemed unchangeable.”
For this study, scientists experimented with a low-energy, or degenerate, Fermi gas of thousands of strontium atoms. Using these quantum gases, the team created the latest atomic clocks.
All the atoms’ properties are restricted to specific values or quantized in these low-temperature Fermi gases. As a result, the atoms avoid each other by keeping a minimum distance between pairs.
Then, by using blue light, the atoms were excited. Later on, the team measured the resulting photon radiation along with different directions. By setting up specific conditions, the team reduced photon emission along a narrow scattering angle by 50%. In this case, an atom prepared in the excited state would, on average, remain in this state 10% longer than usual.
At first, they received a naturally exciting lifetime of atoms of five nanoseconds. As this lifetime is too short to measure, scientists used photon scattering as an indirect indicator.
Ye said, “Future experiments using different energy levels in the atoms or denser and even colder gases could extend excited states for longer periods or even block decay entirely.”
At last, by exciting only a small number of atoms, the team collected the emitted photons at a narrow-angle concerning the blue excitation beam. By doing so, scientists were able to observe small motion transfers.
Scientists noted, “A large angle would give the atoms too much of a momentum kick, increasing their chances of escape and weakening the blocking effect.”
With this technique, it is possible to quantum-engineer atom-light systems with potential applications.
Some exciting features of the experiment include:
- Making a gas with the lowest possible energy.
- Enabling the purely quantum-mechanical blocking phenomenon to occur.
Journal Reference:
- C. Sanner, L. Sonderhouse, R.B. Hutson, L. Yan, W.R. Milner, and J. Ye. Pauli blocking of atom-light scattering. Science. Published online Nov. 18, 2021. DOI: 10.1126/science.abh3483