Disease due to air pollution is the third-leading cause of death in developing nations. According to an estimate, more than 5 million people worldwide die every year from air pollution exposure.
Exhaust systems, the most generally utilized air-purging gadgets, change over the dangerous gases and toxins delivered by fuel burning into amiable chemicals previously the fumes is discharged into the climate. Additionally, they are inefficient, because the precious metal particles are embedded randomly in the catalytic coating and, therefore, some never come into contact with the pollutants they’re meant to clean.
Having a concern about it, scientists at the Harvard Gazette along with Wyss Institute are building up another sort of reactant covering that is propelled by the honeycomb-like nanostructure of a butterfly’s wing. This fundamental structure makes channels through which air can stream unobstructed, and the exact situation of impetuses on the structure boosts the effectiveness of the catalysis responses while diminishing the measure of valuable metals required by around 80 percent. These coatings can be effectively incorporated into the current $20 billion exhaust system industry, and their lower cost could broaden the market for exhaust systems to home use in bringing down pay nations.
The research, led by Joanna Aizenberg, a Core Faculty member of the Wyss Institute, is described in a series of papers published in Advanced Materials, Advanced Functional Materials, and Chemistry – A European Journal. Aizenberg is also the Amy Smith Berylson Professor of Materials Science and Professor of Chemistry & Chemical Biology.
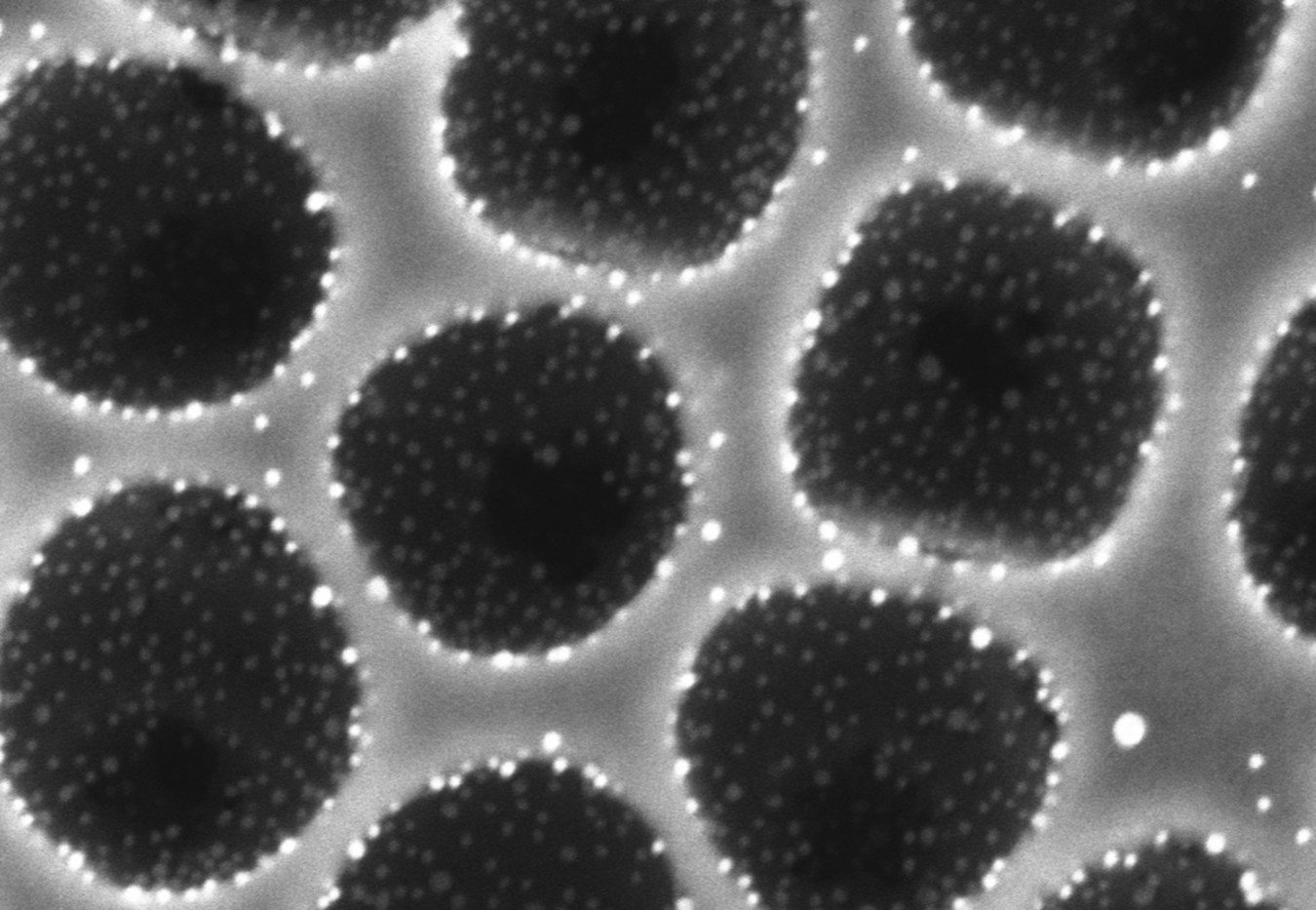
To make these structures, the analysts presented a co-gathering strategy in which modest, circular particles and framework forerunners are kept all the while from a solitary blend to create imperfection free movies over centimeter scales. The specialists showed this procedure with broadly utilized reactant materials, including titania, alumina, and zirconia, joining different mono-and multi-metallic nanoparticles.
The method is relatively simple: First, the catalytic nanoparticles attach to the colloids through various kinds of chemical and physical bonding. Coated with nanoparticles, the colloids are next placed into a matrix precursor solution and allowed to self-assemble into the desired pattern, which can be controlled by confining the assembly within a certain shape.
Lastly, the colloids are removed so that a structured network that is decorated with nanoparticles partially embedded inside the matrix is formed. This hierarchical porous architecture with firmly attached catalytic sites maximizes the surface area for the catalytic reaction and enhances the robustness of the catalyst.
Tanya Shirman said, “Our synthetic platform allows us to take the components of the assembly and form a fully interconnected, highly ordered porous microarchitecture, in which catalytic nanoparticles are uniquely incorporated. This provides exceptional mechanical, thermal, and chemical stability as well as high surface area and full accessibility to diffusing reactants.”
Aizenberg said, “The technology developed in my lab is particularly promising for bridging the gap between state-of-the-art R&D and real-world applications. Due to its modular design and tunability, this framework can be used in various fields from the synthesis of important chemical products to pollution abatement. Our results clearly show that we are now able to create better catalysts, use less precious metal and improve known catalytic processes.”
The study is described in a series of papers published in Advanced Materials, Advanced Functional Materials, and Chemistry – A European Journal.
