According to a new theory developed by Brown scientists stretching or compressing metal, catalysts can make them perform better. Applying a strain to metal catalysts — either compression or tension — can in some cases change the way they perform.
Scientists suggest that the findings could provide new design possibilities for new catalysts with new capabilities.
Catalysts optimize chemical reactions. A catalytic converter on a car, for example, uses metal catalysts to pluck toxins out of exhaust fumes. There’s additional interest in utilizing catalysts to change over carbon dioxide into fuels, make composts from environmental nitrogen and drive responses in energy unit autos.
Andrew Peterson, an assistant professor in Brown’s School of Engineering said, “Strain is a really hot topic in catalysis right now. We’ve started seeing things happening under strain that isn’t easily explained by the traditional theory of how catalysts work. That got us thinking about an alternative framework for this question.”
Catalysts work by adsorption process that breaks chemical bonds of the reactant molecules, enabling various stages of a chemical reaction to take place on the metal’s surface. Once it completes all reactions, the final product is released from the catalyst through the reverse process, called desorption.
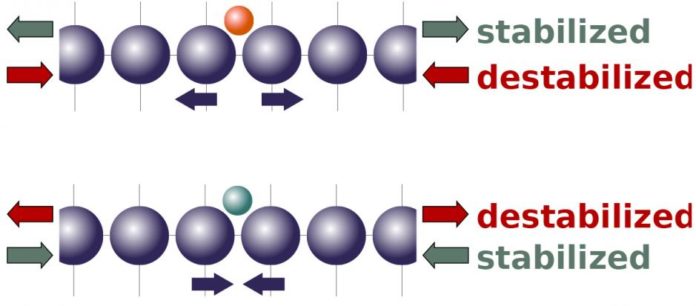
According to the latest theory, the tensile strain should increase reactivity, while compression should reduce it. The customary hypothesis depicts things on the level of electrons and electron groups. The new hypothesis zooms out a bit, concentrating rather on the mechanics of how particles connect with an impetus’ nuclear cross section.
Scientists showed that the molecules bound to an catalysts’ surface will have a tendency to either push atoms in the cross-section separated or pull them closer together, relying on the attributes of the atoms and the coupling destinations.
The different forces delivered by molecules have fascinating implications for how outside strain should influence an impetus’ reactivity. It recommends that strain, which extends an impetus’ nuclear cross section, should make an impetus more responsive to atoms that normally need to push the grid separated. In the meantime, pressure should diminish reactivity for particles that need to pull the cross section together. Pressure — crushing the grid — has a reverse impact.
Peterson said, “Scaling relations mean that, under normal circumstances, when you increase a catalyst’s reactivity for one chemical, it increases the reactivity of other chemicals as well. Similarly, if you decrease reactivity for one chemical, you decrease it for others.”
This new theory suggests that strain can break those scaling relations — enabling a catalyst to simultaneously bind one chemical more tightly and another more loosely, depending on the chemical’s natural interaction with the catalyst’s atomic lattice and the way that the strain field is engineered on the catalyst surface.
Peterson said, “Now you can start to think about really fine tuning catalysts to perform better throughout different reaction steps. That could dramatically improve a catalyst’s performance, depending on the chemicals involved.”
According to Paterson, the work will provide that catalysis community with a new way of thinking about strain. Thus, whenever people design new catalysts, they can think of ways to better harness these strain effects.
The theory, described in the journal Nature Catalysis.