In today’s date, scientists are struggling to organize cells into complex 3D arrangements that our bodies can master independently. There are two major problems: 1. to use a biologically compatible 3D scaffold in which cells can grow and 2. to decorate that scaffold with biochemical messages in the correct configuration to trigger the formation of the desired organ or tissue.
Scientists from the University of Washington turned this into reality. They come up with a method that uses protein-based biochemical messages to modify naturally occurring biological polymers. This naturally affects the cell’s behavior.
This new approach also uses a near-infrared laser to trigger the chemical adhesion of protein messages to a scaffold made from biological polymers such as collagen, a connective tissue found throughout our bodies.
Senior author Cole DeForest, a UW associate professor of chemical engineering and bioengineering, said, “Mammalian cells responded as expected to the adhered protein signals within the 3D scaffold. The proteins on these biological scaffolds triggered changes to messaging pathways within the cells that affect cell growth, signaling, and other behaviors.”
“These methods could form the basis of biologically based scaffolds that might one day make functional laboratory-grown tissues a reality.”
“This approach provides us with the opportunities we’ve been waiting for to exert greater control over cell function and fate in naturally derived biomaterials — not just in three-dimensional space but also over time. Moreover, it makes use of exceptionally precise photochemistry that can be controlled in 4D while uniquely preserving protein function and bioactivity.”
This is the first method that spatially controls cell function inside naturally occurring biological materials.
Previously, several research groups have developed light-based methods to modify synthetic scaffolds with protein signals. But natural biological polymers can be a more attractive scaffold for tissue engineering because they innately possess biochemical characteristics that cells rely on for structure, communication, and other purposes.
DeForest said, “A natural biomaterial like collagen inherently includes many of the same signaling cues as those found in native tissue. In many cases, these types of materials keep cells ‘happier’ by providing them with similar signals to those they would encounter in the body.”
Scientists tested their method with two types of biological polymers: collagen and fibrin. They assembled each into fluid-filled scaffolds known as hydrogels.
The signals that the team added to the hydrogels are proteins, one of the main messengers for cells. Proteins come in many forms, all with their unique chemical properties. As a result, the researchers designed their system to employ a universal mechanism to attach proteins to a hydrogel — the binding between two chemical groups, an alkoxyamine and an aldehyde.
Before hydrogel is assembled, scientists the collagen or fibrin precursors with alkoxyamine groups, all physically blocked with a “cage” to prevent the alkoxyamines from reacting prematurely. The cage can be removed with an ultraviolet light or a near-infrared laser.
Using methods previously developed in DeForest’s laboratory, the researchers also installed aldehyde groups to one end of the proteins they wanted to attach to the hydrogels. They then combined the aldehyde-bearing proteins with the alkoxyamine-coated hydrogels and used a brief pulse of light to remove the cage covering the alkoxyamine.
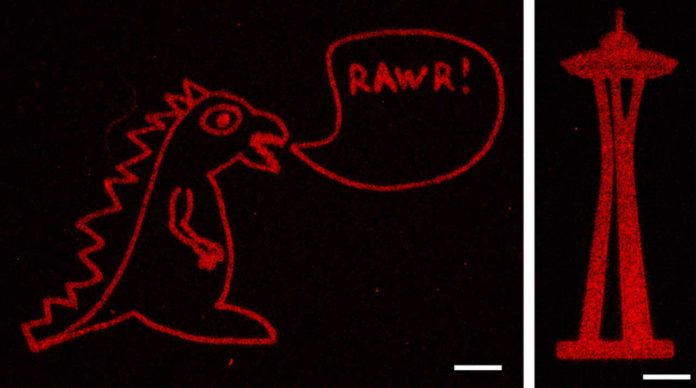
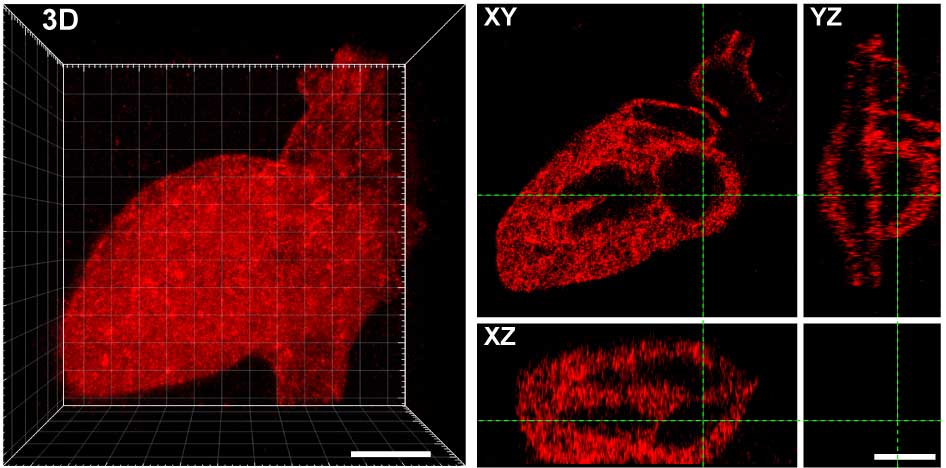
The exposed alkoxyamine readily reacted with the aldehyde group on the proteins, tethering them within the hydrogel. The team used masks with patterns cut into them and changes to the laser scan geometries to create intricate designs of protein arrangements in the hydrogel — including an old UW logo, Seattle’s Space Needle, a monster, and the 3D layout of the human heart.
The tethered proteins were fully functional, delivering desired signals to cells. When loaded onto collagen hydrogels bearing a protein called EGF, which promotes cell growth, rat liver cells showed signs of DNA replication and cell division. In a separate experiment, the researchers decorated a fibrin hydrogel with patterns of a protein called Delta-1, which activates a specific pathway in cells called Notch signaling. When they introduced human bone cancer cells into the hydrogel, cells in the Delta-1-patterned regions started Notch signaling, while cells in areas without Delta-1 did not.
DeForest said, “These experiments with multiple biological scaffolds and protein signals indicate that their approach could work for almost any type of protein signal and biomaterial system.”
“Now, we can start to create hydrogel scaffolds with many different signals, utilizing our understanding of cell signaling in response to specific protein combinations to modulate critical biological function in time and space.”
Journal Reference:
- “Photopatterned biomolecule immobilization to guide three-dimensional cell fate in natural protein-based hydrogels,” PNAS (2020). DOI: 10.1073/pnas.2014194118