The delivery of therapeutic molecules is a significant bottleneck for medicine. There is a need for a deep bench of options to get these powerful new therapies into the right cells in the body.
In a new study, researchers learned how nature transports proteins. Based on that, they came up with a platform to help address this gap.
A new protein delivery strategy that functions in both human and animal cells has been created by researchers at the McGovern Institute for Brain Research at MIT and the Broad Institute of MIT and Harvard. The system may be programmed to deliver various proteins, such as those used in gene editing, to different cell types. The method might be a secure and effective approach to administering cancer and gene therapy.
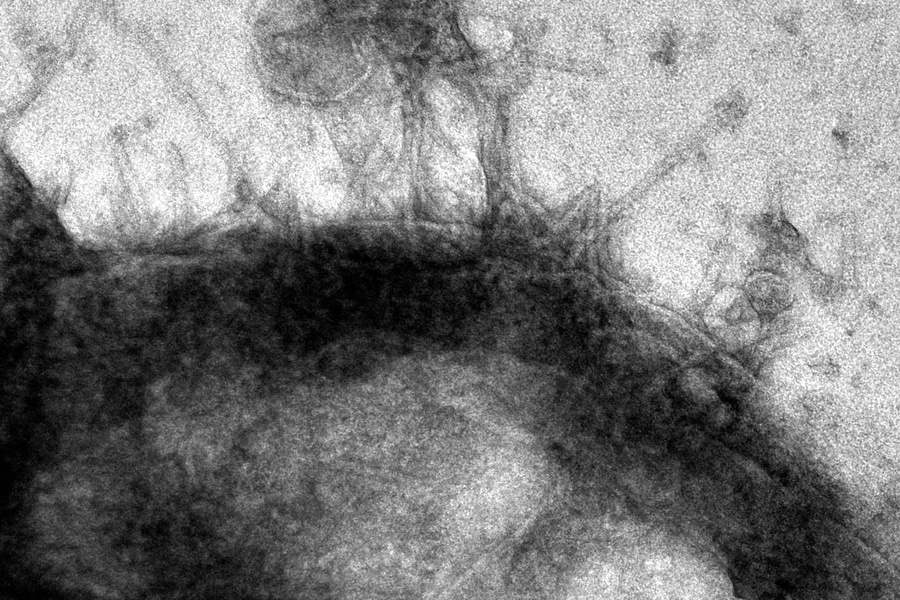
Researchers took advantage of a tiny syringe-like injection structure produced by a bacterium that naturally binds to insect cells and injects a protein payload into them. Using the AI tool AlphaFold, they engineered these syringe structures to deliver several useful proteins to both human cells and cells in live mice.
Endosymbiotic bacteria have evolved intricate delivery systems that enable these organisms to interface with host biology. One example, the extracellular contractile injection systems (eCISs), are syringe-like macromolecular complexes that inject protein payloads into eukaryotic cells by driving a spike through the cellular membrane.
Tail fibers that detect particular receptors on the cell surface and latch on are located on the exterior of one end of the eCIS. Earlier studies have demonstrated that eCISs can naturally target insect and mouse cells. Kreitz hypothesized that it could be possible to adapt eCISs to transport proteins to human cells by re-engineering the tail fibres to attach to different receptors.
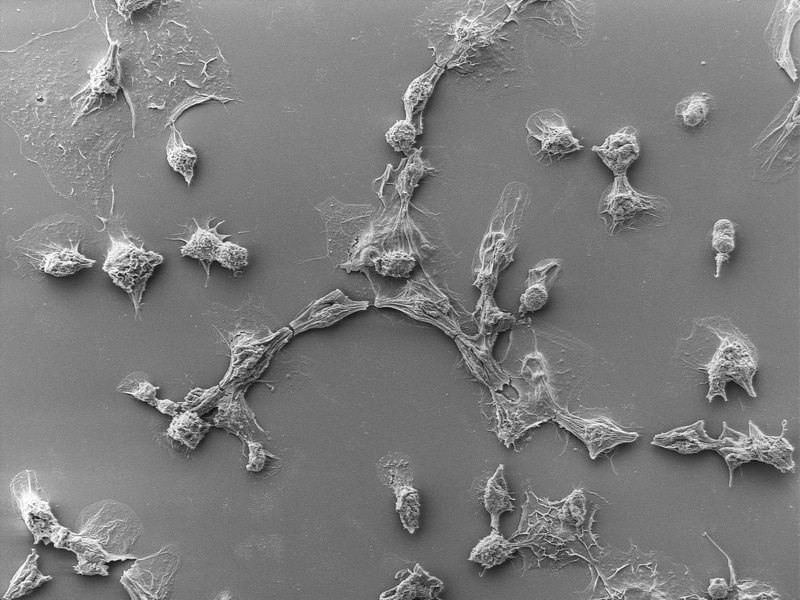
Researchers then used AI to redesign the tail fibers of an eCIS produced by Photorhabdus bacteria to bind to human cells. This re-engineering allows scientists to trick the syringe into delivering a protein of their choosing, in some cases with remarkably high efficiency.
The researchers created eCISs that specifically targeted cancer cells expressing the EGF receptor and demonstrated that they nearly wholly eliminated the target cells while having little effect on cells lacking the receptor. Despite the fact that effectiveness partly depends on the receptor the system is intended to target.
The researchers also utilized an eCIS to transfer proteins to the brains of living mice securely, and they found that this method didn’t trigger any immune responses. This suggests that eCISs may one day be used to deliver gene treatments to people without risk.
Joseph Kreitz, the study’s first author, a graduate student in biological engineering at MIT, and a member of Zhang’s lab, said, “the eCIS system is versatile, and the team has already used it to deliver a range of cargoes, including base editor proteins (which can make single-letter changes to DNA), proteins that are toxic to cancer cells, and Cas9, a large DNA-cutting enzyme used in many gene editing systems.”
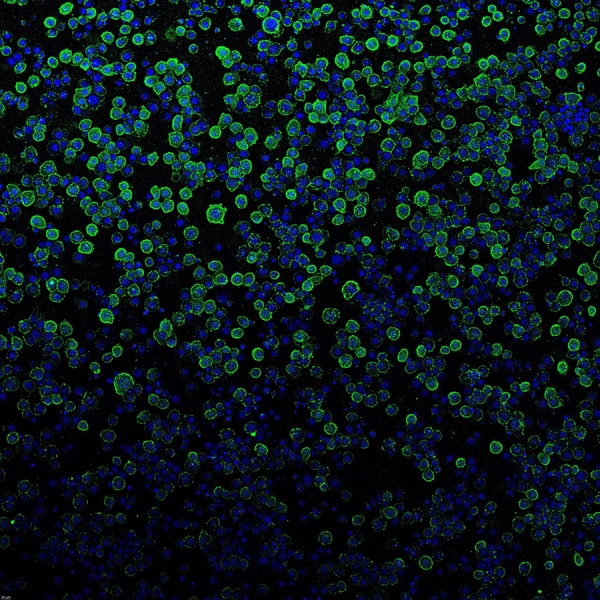
“In the future, researchers could engineer other components of the eCIS system to tune other properties or to deliver other cargoes such as DNA or RNA. He also wants better to understand the function of these systems in nature.”
“We and others have shown that this type of system is incredibly diverse across the biosphere, but they are not very well characterized. And we believe this type of system plays important roles in biology that are yet to be explored.”
Journal Reference:
- Kreitz, J., Friedrich, M.J., Guru, A. et al. Programmable protein delivery with a bacterial contractile injection system. Nature (2023). DOI: 10.1038/s41586-023-05870-7
