An Indian Scientist has expanded a new, freeware software that can track the development of embryo and development of neuronal cells all over the body in the worm. Now it is available to scientists and doctors as well. Even if, scientists have detected number of important proteins that decide how neurons conducts during formation of the brain, it is broadly unknown how these whole proteins collaborate in a living organism.
Hari Shroff, head of National Institute of Biomedical imaging and Bioengineering (NIBIB) research team said, “Understanding why and how neurons form and the path they take to reach their final destination could one day give us valuable information about new proteins and other molecular factors interact during neural development.”
In their project, this new technology will be central to create 4D neuro-developmental “worm atlas” which attempts list out the formation of the worm nervous system.
This list will be the first absolute view of how an entire nervous system develops.
According to Shroff, “it will be helpful in understanding the fundamental mechanisms by which all nervous systems, including ours, accumulating including ours.”
He is also expecting that some of the approaches developed like, from multiple embryones, the approach is taken to combine neuronal data that can be applied to additional model entities alongside the worm.
Known as C elegans, the worm has 302 neurons and out of them, 222 are developed while the worm is still an embryo. The worm has its own self-versions of many similar proteins which are used for direct development of the brain in more complex entities like mice, humans, and flies.
Shroff claimed, “We still don’t understand neuro-development even in the context of the humble worm but we are using it as a simple model of how these factors work together to drive the development of worm brain and neuronal structure.”
He then said, “We are in hope that by doing this, some of the lessons will transfer all the way up to humans.”
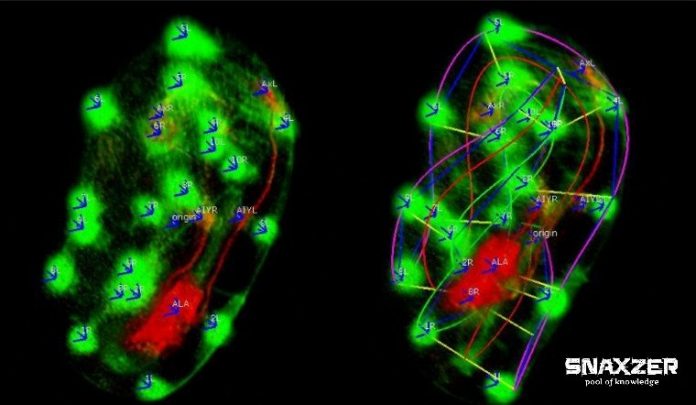
At Memorial Sloan-Kettering Institute in US, in partnership with Daniel colon-Ramus at Yale University and Zhirong Bao, the team from NIBIB have developed new microscopes which improves the speed and resolution, allowing it to record the embryogeny process inside worm through too much display of light while still getting the boldness needed to see individual cells clearly.
The researcher said, “The problem was during development, the worm starts ‘twitch’ moving around inside the egg. The folding and twisting make it hard to track cells and parse out movement.”
After all, while looking at two-dimensional image especially of folded worm, it is challenging to terminate where a neuron is in 3D space.
With Fluorescent protein, scientists have made individual cells to act as a marker in the embryo. When a microscopic image of these cells is delivered to the programs, the computer will detect each cell and uses the information to create a model of the worm, which it then computationally ‘untwists’ to generate a perpendicular image.
This program also approves a user to check the accuracy of the computer model and if any mistakes are found, they may edit it.
Ryan Christensen, a postdoctoral fellow at NIBIB said, “In addition, the user can also mark cells or structures within the warm embryo they want the program to track, allowing the users to follow the position of a cell as it moves and grows in developing an embryo.”
“This feature could help the scientists to understand how certain cells develop into neurons, as opposed to other types of cells, and what factors influence the development of the brain and neuronal structure”, he added.