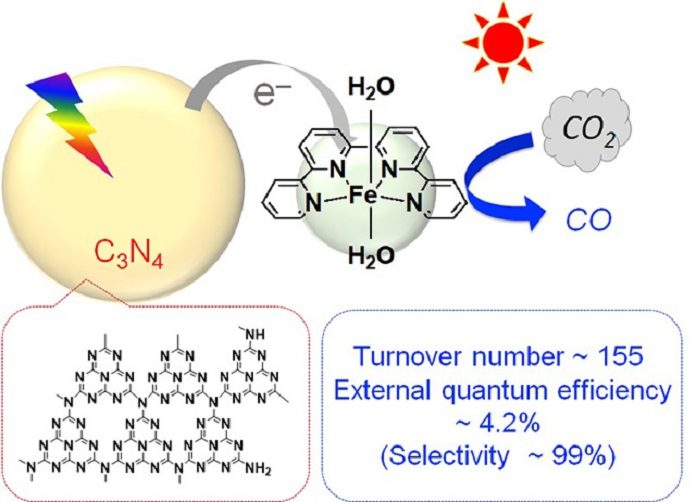
Scientists at the Tokyo Institute of Technology in collaboration with Université PARIS DIDEROT and CRNS have discovered a new method to reduce CO2 to CO1. They fused carbon nitride, an organic semiconductor, with a complex made of iron and organic materials and using it as a photocatalyst, they succeeded in turning CO2 into a resource at high efficiency under the condition of exposure to visible light at ordinary temperature and pressure.
Scientists combined the organic semiconductor carbon nitride3, made of carbon and nitrogen, with an iron complex and using it as a photocatalyst. Doing this enabled them to reduce carbon dioxide (CO2) to carbon monoxide (CO) at high efficiency.
When exposing this process directly to the visible light, the carbon nitride absorbs visible light and drives the migration of electrons from the reducing agent to the iron complex, the catalyst.
The iron complex uses that electrons to reduce CO2 to CO. The turnover number, the external quantum efficiency, and the selectivity of CO2 reduction—performance indicators for the formation of CO—reached 155, 4.2%, and 99%, respectively. These values are almost the same as when precious metal or rare metal complexes are used, and about ten times more than photocatalyst reported so far using base metals or organic molecules.
Scientists reported that this is the first study that demonstrates CO2 can be reduced into a resource efficiently using sunlight as the energy source.
Now scientists are further planning to improve their function as a photocatalyst and to succeed in infusing them with oxidation photocatalysts which can use water, which exists abundantly on Earth and is inexpensive, as a reducing agent.
Professor Osamu Ishitani, Associate Professor Kazuhiko Maeda, research staff Ryo Kuriki and others of Tokyo Tech, with the support of JST (Japan Science and Technology Agency)’s Strategic Basic Research Programs (CREST Establishment of Molecular Technology towards the Creation of New Functions) for international collaborative research projects, performed collaborative research with the research group of Professor Marc Robert of Université PARIS DIDEROT and CRNS.
Scientists reported their study in the Journal of American Chemical Society.