A new study has shed light on the way heart valve grows and find their shapes in embryos. Bioengineers have discovered a new mechanism that drives the growth of heart valves in zebrafish embryos.
The National Institute of Health and Medical Research study in France, University of Strasbourg, and Imperial College London could also help understand why some valves don’t develop properly, opening up a way towards new treatment.
In humans, blood passes through a valve before leaving each chamber of the heart. The valves prevent the backward flow of blood.
Heart valves open and shut as the heart muscle contracts and relax. This lets blood flow into the ventricles and atria at alternate times.
When heart valves fail to open and close correctly, the damage to the heart can be severe. Some people are born with diseased heart valves – known as congenital heart valve defects. But, how valves grow in embryos is poorly understood.
To study their development, bioengineers used zebrafish models to identify the mechanosensitive process that plays a vital role in the development of atrioventricular valves.
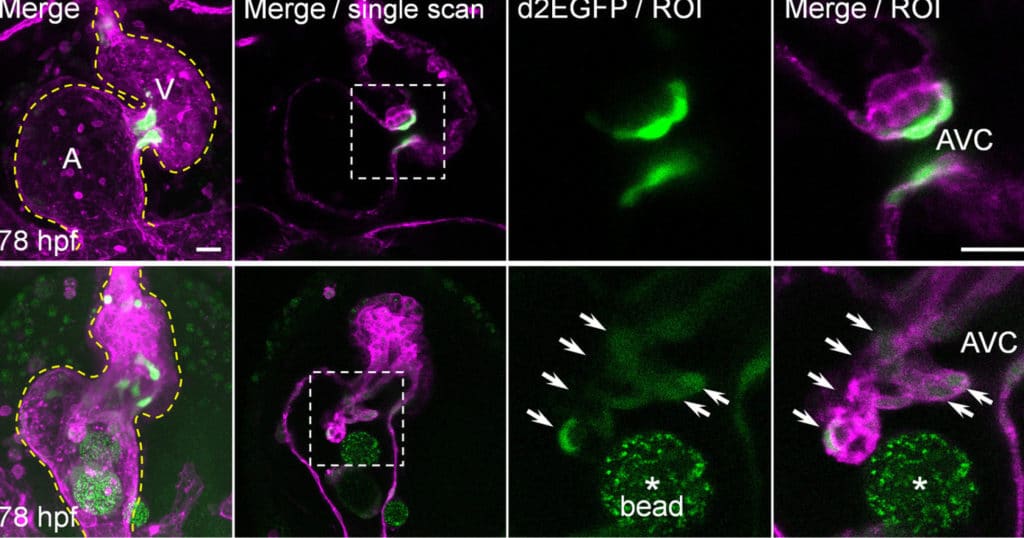
They found that alongside mechanisms already known to generate heart valve tissue, another mechanism works in parallel to determine the shape and function of the atrioventricular canal (AVC), from which the atrioventricular valves develop. Further studying the role of mechanical forces in congenital valve defects could eventually help understand how to prevent and treat them.
Co-author Dr. Julien Vermot of Imperial’s Department of Bioengineering said: “Mechanical forces in embryos may determine the shape of many organs in the body. We have discovered a pathway that’s crucial for heart valve development, and our findings could help to inform the future prevention and treatment of diseases of the heart valves.”
In particular, scientists studied the roles of calcium ions (Ca2+) and adenosine triphosphate (ATP). Scientists found that some mechanical forces are at play that activates these signaling molecules.
They found that these factors contribute to mechanisms in heart valve cell creation that are driven by the mechanical forces activated by the beating heart.
Dr. Vermot added: “This work further unravels how mechanical forces can influence tissue remodeling in developing organs.”
Scientists noted, “The findings could help us better understand how mechanical forces affect cell differentiation and what their role might be in producing malformed valves. They could lead to drugs, treatments, and even be used to help growing replacement valve for patients with valve defects.”
The research team is looking forward to determining how the pathway interacts with others and how their findings might translate into tissue engineering and other organisms like mice and humans.
Journal Reference:
- HAJIME Fukui et al. Bioelectric signaling and the control of cardiac cell identity in response to mechanical forces. DOI: 10.1126/science.abc6229