Scientists at the University of Geneva (UNIGE), Switzerland have developed a new molecular switch that is capable of binding metals present in its environment. The switch comes in the form of sensor that forms a 3D structure whose molecules are asymmetric.
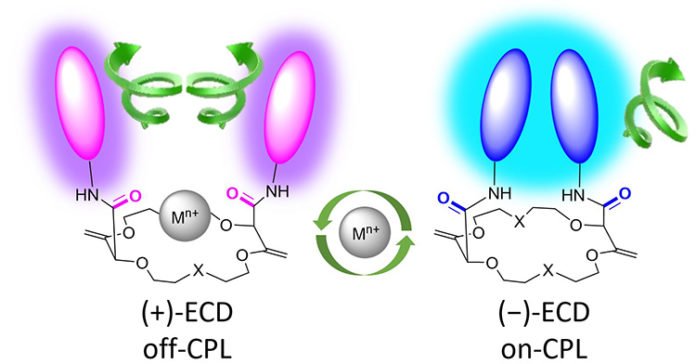
These molecules comprise a ring and two luminescent arms that emit a particular type of light in a process called Circular Polarized Luminescence (CPL), and selectively detections, such as sodium. The luminescent arms mimic as a light bulb that emits light during the metal binding process depending on the presence of a positively charged ion, a metal cation.
Jérôme Lacour, Dean of the Faculty of Science at UNIGE and Ordinary Professor in the Department of Organic Chemistry said, “These molecules can be contrasted with small locks: when they are prepared to work and identify the presence of metals, they produce a specific sort of light (circularly enraptured). At the point when a metal particle is embedded, it follows up on them like a key, the lock geometry changes, and the light disappears.”
These “locks” are comprised of two sections: a ring (a crown ether) that can surround metal particles, for example, sodium, and two bent arms that stretch out from the edge and act like lights, enabling analysts to “see” regardless of whether metal particles are available or not. Without metal particles, the two arms are near one another and produce an extreme enraptured glow. At the point when a metal particle is embedded, the atom changes geometry. The arms move separated and quit discharging light. You can likewise “remove the key” by including a forager particle.
The luminescence of the two arms is then totally recovered. This on/off capacity can be rehashed more than a few on/off cycles, making these atoms viable and simple to read. The atom in this manner carries on like a switch; it gives a visible flag whose practical applications could be various, beginning with the identification of metals in the environment.
Jérôme Lacour said, “Our system is based on a small molecule of carbon, oxygen, and hydrogen, and has the advantage of reacting to simple and naturally abundant cations like sodium. Despite the functional complexity of the molecules, this new type of sensor can easily be assembled through only two synthetic steps, which could be established after three years of research.”
This research can be read about in Chemical Science.