Neurons communicate with one another at junctions called synapses. When calcium ions move into “active zones,” which are populated with vesicles containing chemical messages, they begin to “communicate.” Vesicles “fuse” to the presynaptic neurons’ outer membranes due to the electrically charged calcium, releasing their communication chemical cargo to the postsynaptic cell.
A new study by the Picower Institute for Learning and Memory at MIT reveals how neurons set up and sustain this vital infrastructure.
Calcium channels are a crucial part of the engine on the presynaptic side that transforms electrical signals to chemical synaptic transmission since they are the primary determinant of calcium influx, which then causes vesicle fusion. However, how they accumulate in active zones was unclear.
This new study offers clues on how active zones accumulate and regulate the abundance of calcium channels.
Troy Littleton, a senior author of the new study and Menicon Professor of Neuroscience in MIT’s departments of Biology and Brain and Cognitive Sciences, said, “Modulation of the function of presynaptic calcium channels is known to have significant clinical effects. Understanding the baseline of how these channels are regulated is important.”
Are calcium channels essential for active zones to develop?
Scientists wanted to determine the answer to this question in larvae. It should be noted that the fly calcium channel gene (called “cacophony,” or Cac) is so important that they can’t live without it.
Instead of knocking out Cac across the entire fly, scientists employed a technique to eliminate Cac in just one population of neurons. They demonstrated that active zones regularly develop even without Cac by doing this.
They also used another technique that artificially prolongs the larval stage of the fly. They found that given extra time the active zone will continue to build up its structure with a protein called BRP, but Cac accumulation ceases after the normal six days.
It was also found that moderate increases or decreases in the supply of available Cac in the neuron did not affect how much Cac ended up at each active zone. To their surprise, they found that although the number of Cac scaled with the size of each active zone, it scarcely changed if they significantly reduced the BRP in the active zone. In fact, the neuron appeared to establish a constant cap on the amount of Cac present for each active zone.
MIT postdoc Karen Cunningham said, “It was revealing that the neuron had very different rules for the structural proteins at the active zone like BRP that continued to accumulate over time, versus the calcium channel that was tightly regulated and had its abundance capped.”
Besides Cac supply or changes in BRP, other factors must also regulate Cac levels so tightly. They turned to alpha2delta.
Genetically manipulating the expression of its amount, scientists found that alpha2delta levels directly determined how much Cac accumulated at active zones. Further experiments also revealed that the neuron’s overall Cac supply monitors the alpha2delta’s ability to maintain Cac levels.
It suggests that instead of controlling Cac amount at active zones by stabilizing it, alpha2delta likely functioned upstream, during Cac trafficking, to supply and resupply Cac to active zones.
Using two different techniques, they observed this resupply. They also generated measurements of it and its timing.
Cunningham chose a moment after a few days of development to image active zones and measured Cac abundance to ascertain the landscape. Then she bleached out that Cac fluorescence to erase it. After 24 hours, she visualized Cac fluorescence anew to highlight only the new Cac that was delivered to active zones over that 24 hours.
She observed that Cac was delivered throughout almost all active zones that day. Still, that one day’s work was, in fact, insignificant in comparison to the accumulation from earlier days. She also saw that larger active zones accumulated more Cac than smaller ones. Additionally, there was hardly any new Cac delivery in the altered alpha2delta fly models.
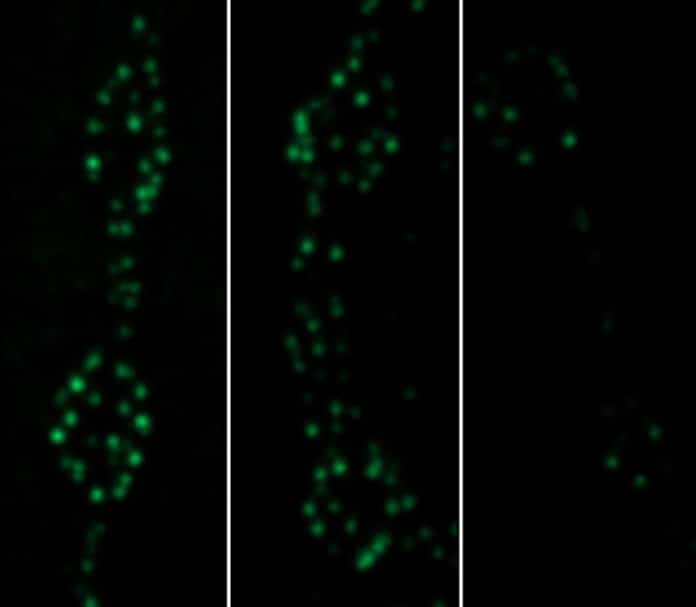
The next task was to determine at what pace Cac channels are removed from active zones. To do so, scientists used a staining technique with a photoconvertible protein called Maple tagged to the Cac protein. This allowed them to change the color with a flash of light at her chosen time.
Doing so shows how much Cac accumulated by a specific time (shown in green) and then flashes the light to turn that Cac red. After five days, nearly 30 percent of the red Cac had been replaced with new green Cac. This Cac turnover stopped when the Cac delivery levels were reduced by mutating alpha2 delta or reducing Cac biosynthesis.
Cunningham said, “That means a significant amount of Cac is turned over each day at active zones and that the turnover is prompted by new Cac delivery.”
Littleton said, “Now that the rules of calcium channel abundance and replenishment are clear, I want to know how they differ when neurons undergo plasticity — for instance when new incoming information requires neurons to adjust their communication to scale up or down synaptic communication.”
“I’m also eager to track individual calcium channels as they are made in the cell body and then move down the neural axon to the active zones, and he wants to determine what other genes may affect Cac abundance.”
Journal Reference:
- Karen L Cunningham, Chad W Sauvola, Sara Tavana, J Troy Littleton. Regulation of presynaptic Ca2+ channel abundance at active zones through a balance of delivery and turnover. Neuroscience. DOI: 10.7554/eLife.78648