Most human cancers are caused by the cancer gene MYC, which makes it the “Mount Everest” of cancer research. Many cancer patients could benefit from the MYC medication.
Researchers from the University of Münster, the Max Planck Institute of Molecular Physiology in Dortmund, and the Wertheim UF Scripps Institute in Florida have collaborated to create materials that break apart the RNA of MYC.
The recently developed RNA degrader technique may also pave the way for other difficult-to-treat disorders. Ribotac destroys the RNA of cancer genes. Matthew Disney and his colleagues at The Wertheim UF Scripps Institute have spent the last 15 years identifying several conserved, druggable RNA structures and demonstrating in animals that targeting RNAs may kill cancer tumors and improve other disorders.
Certain proteins play a significant role in cancer cells’ growth, division, and spread, making them attractive targets in cancer research and treatment. However, most cancer-promoting proteins have survived all attempts by scientists to reverse their negative effects.
Cancer gene targeting’ RNA provides an alternative approach to treating disorders whose crucial protein structures cannot be addressed by drugs. Beyond its role as a protein-coding template, RNA regulates the homeostasis in healthy and cancer cells via crucial interactions with various cellular components.
However, the structures of RNAs are so complex and dynamic that many in the pharmaceutical industry dismissed attempts to develop RNA-directed treatments as futile.
Matthew Disney and his colleagues at The Wertheim UF Scripps Institute have identified numerous conserved, druggable RNA structures over 15 years. Disney’s team created their chemical libraries and demonstrated in mice that targeting RNAs can eradicate cancer tumors and improve other diseases such as ALS and myotonic dystrophy.
The idea for the collaboration originated in 2016 during a scientific meeting in Spain when Waldmann and Disney shared notes after a session. Both agreed that using medications to target RNA was an interesting new technique to treat incurable diseases, but it was still early. Nothing like that was available on the market.
In 2018, two members of Disney’s team visited Waldmann’s group at the Max Planck Institute of Molecular Physiology in Dortmund to search for suitable RNA-binding candidates using their screening methodologies and chemical libraries. Disney stated that the findings exceeded his expectations: they discovered that the universe of known, druggable RNA structures had grown considerably.
Disney’s team discovered 2,000 novel RNA structures capable of binding drug-like small molecules and six new chemotypes capable of binding RNA.
Some of the chemicals in the library were created by the University of Münster’s team of organic chemist Frank Glorius.
Glorius said, “We develop molecules that exhibit sought-after function in diverse biological areas, like the compounds in the library, originally designed to specifically influence biology in cell membranes. But we would have never expected them to bind RNA structures.”
The compounds most effective at binding to the cancer-related RNAs MYC, JUN, and microRNA-155 were those generated from imidazole. This substance is frequently found in food and medicine.
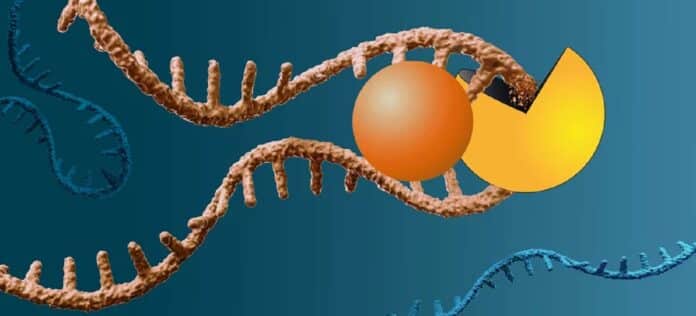
Disney’s team created a novel way to remove disease-causing RNA segments by attaching a chemical fishing hook to the molecules designed to snag the cell’s RNA recycling enzymes.
The RNA recycling enzyme cut up the RNA to which the drug molecule was attached, thus preventing disease-causing proteins from forming. Their hybrid molecule became known as a RiboTAC, which stands for ribonuclease targeting chimera.
Disney said, “With the degrader added, we started seeing these ‘undruggable’ cancer RNAs reduced by 35, 40, 50 percent or more. This caused cancer cells to die and cleared tumors in mouse-based breast cancer studies that spread to the lungs; This study shows that compound classes inspired by natural products provide a rich, new source for targeting RNA disease targets in general. Of course, we are developing them further, following our concept of pseudo-natural products.”
He added, “Because the strategy is so novel, it will take several years of additional work before the innovations reach patients via a clinical trial. This is a long road to take, a marathon run.”
One of the most important and challenging drug targets is MYC. Its activation could impact 70% or more of all malignancies in humans. It has the power to control the expression of numerous other genes.
It impacts cell proliferation and even the crucial apoptosis process, which causes damaged cells to commit suicide. It has an impact on blood vessel expansion as well as the mechanism by which broken DNA is repaired. MYC is overexpressed in many malignancies, which causes cells to proliferate and divide too quickly.
Waldmann said, “The compounds are a good starting point and show us where to go to build small molecules, RNA-targeting medicines that could eventually treat patients with diseases like aggressive cancers that currently have poor or no options.”
More than 20 different cancer forms, including glioblastoma, breast, prostate, lung, colorectal cancer, and others, have been linked to activating another cancer gene called JUN. MIR155 is a short RNA gene that promotes inflammation and the development and spread of numerous diseases.
Disney said, “This new data also shows us that this approach could have many other disease applications.”