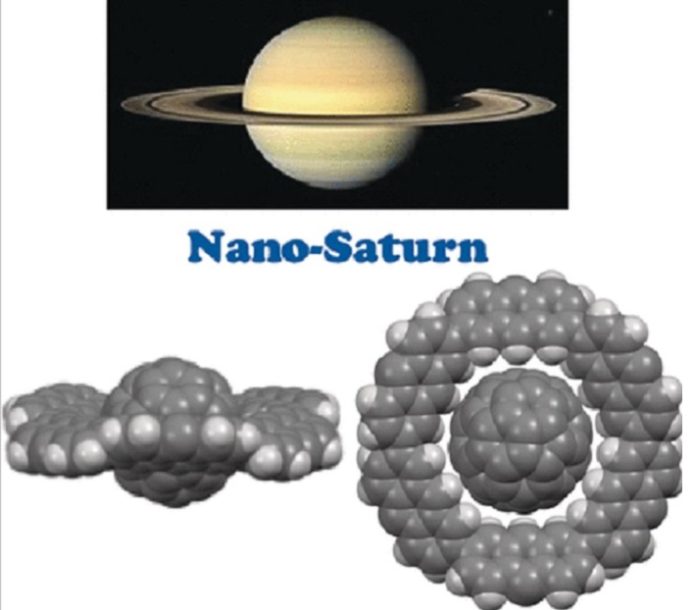
Saturn, 2nd largest planet in our solar system, has a special characteristic ring. Japanese scientists have now synthesized a molecular “nano-Saturn,” which comprises a spherical C(60) fullerene as the planet and a flat macrocycle made of six anthracene units as the ring.
According to scientists, the ring must have a rigid, circular form and must hold the molecular sphere firmly in its midst. Its Fullerenes are ideal candidates for the nano-sphere.
They are made of carbon particles connected to a system of rings that shape an empty sphere. The most popular fullerene, C(60), comprises 60 carbon particles organized into 5- and 6-membered rings like the leather patches of a great soccer ball.
The electrons in their double bonds, known as the π-electrons, are in a sort of “electron cloud”, ready to uninhibitedly move about and have restricting associations with different particles, for example, a macrocycle that likewise has a “cloud” of pi-electrons. The alluring interactions between the electron cloud enable fullerenes to lodge in the holes of such macrocycles.
A progression of such complexes has beforehand been integrated. As a result of the places of the electron mists around the macrocycles, it was beforehand just conceivable to make rings that encompass the fullerene-like a belt or a tire. The ring around Saturn, however, isn’t like a “belt” or “tire”; it is a flat disc.
Scientists wanted to imitate this at the nanoscale properly. They successfully created a different type of bonding between the “nano-planet” and its “nano-ring”. Rather than utilizing the fascination between the pi-electron clouds of the fullerene and macrocycle, the group working with Shinji Toyota utilized the frail appealing collaborations between the pi-electron cloud of the fullerene and non-π-electron of the carbon-hydrogen groups of the macrocycle.
Scientists particularly used anthracene units, molecules made of three aromatic six-membered carbon rings linked along their edges.
They connected six of these units into a macrocycle whose cavity was the ideal size and shape for a C(60) fullerene. Eighteen hydrogen particles of the macrocycle venture into the center of the cavity. Altogether, their collaborations with the fullerene are sufficient to give sufficiently complex stability, as appeared by computer reenactments. By utilizing X-beam examination and NMR spectroscopy, the group could demonstrate tentatively that they had created Saturn-shaped complexes.
They connected six of these units into a macrocycle whose cavity was the ideal size and shape for a C(60) fullerene. Eighteen hydrogen particles of the macrocycle venture into the center of the cavity. Altogether, their collaborations with the fullerene are sufficient to give sufficiently complex stability, as appeared by computer reenactments. By utilizing X-beam examination and NMR spectroscopy, the group could demonstrate tentatively that they had created Saturn-shaped complexes.
The scientists reported the study in the journal Angewandte Chemie.
Journal Reference
- Shinji Toyota. Nano-Saturn Supramolecular complex formation: Anthracene macrocycle and C60 fullerene. Angewandte Chemie International Edition. DOI: 10.1002/anie.201804430