The oxidation of aromatic hydrocarbons is basically vital for delivering an extraordinary assortment of valuable natural exacerbates that are utilized all through a wide range of ventures. These oxidation forms require the utilization of impetuses and solvents, which are typically ecologically dangerous. Along these lines, finding a dissolvable free oxidation process utilizing nanosized reactant particles has pulled in extensive consideration.
Strangely, sub-nanoscale synergist particles (subnanocatalysts, or SNCs) made out of honorable metals are far superior at their actions in light of the fact that their expanded surface territory and one of a kind electronic state results in great impacts for oxidizing hydrocarbons and furthermore keeps them from getting oxidized themselves. This makes them savvy on the grounds that the measure of metal required for SNCs is lower than for nano-sized impetuses.
Now, a team at Tokyo Tech have created multiple types of SNCs by using dendrimers, which are tree-like spherical molecules that can be used as a template to contain the desired catalysts. These subnano-sized metallic particles are very effective as catalysts for the oxidation of hydrocarbons. These catalysts can be as much as 50 times more effective than well-known Au-Pd bimetallic nanocatalysts.
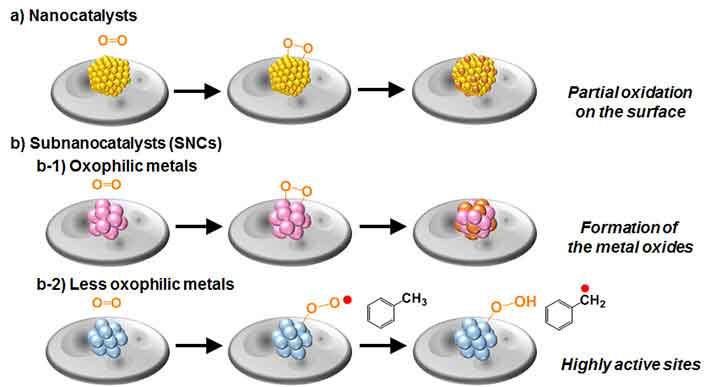
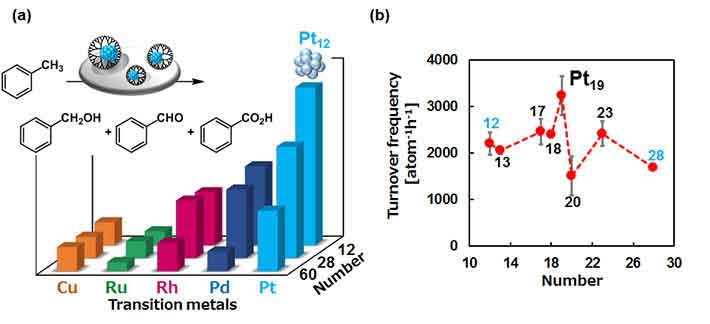
Scientists created different catalysts of various sizes, contingent upon the honorable metal utilized and the number of atoms of each synergist molecule. They contrasted their execution to locate the best noble metal for making SNCs and after that attempted to decide the component behind their high synergist movement. Littler SNCs were observed to be better, while less oxophilic metals, (for example, platinum) were predominant.
After that scientists postulated that the surface of platinum SNCs does not oxidize easily, which makes them reusable and results in the highest catalytic performance of the Pt19 SNC that can be as high as 50 times more effective than the common Au-Pd nanocatalysts.
Dr. Makoto Tanabe at Tokyo Institute of Technology (Tokyo Tech) said, “The development of a more detailed mechanism including theoretical considerations is currently in progress. The applications of such catalysts could greatly contribute for reducing pollution and enhancing our effective use of Earth’s metal resources.”
The study is published in the Angewandte Chemie International Edition.