Gastroesophageal junction (GEJ) adenocarcinoma is often an aggressive cancer, and early disease models to study the molecular basis of this aggressiveness are lacking. Acid reflux, smoking, and Helicobacter pylori bacterial infection of the stomach are well-established risk factors for tumors of the esophagus and stomach. But experts say it’s been challenging to show how cancer begins at the junction of the stomach and the esophagus, partly due to a lack of biologically relevant GEJ-specific early disease models for research.
As no unique model distinguishes GEJ tumors, gastroesophageal cancers are often classified as either esophageal or gastric cancer — not GEJ cancer.
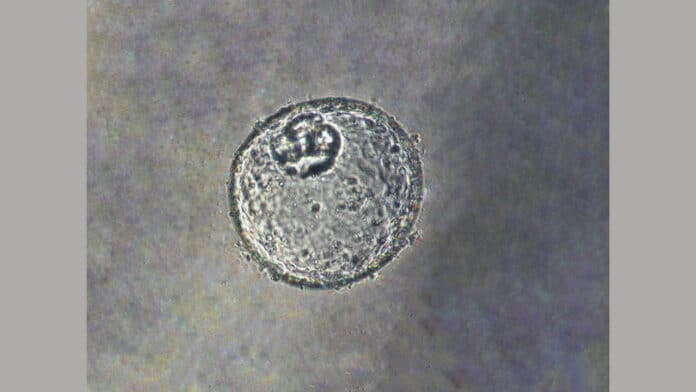
To advance understanding of the early stages of cancer development at the gastroesophageal junction (GEJ), scientists at Johns Hopkins Medicine have created a laboratory-grown three-dimensional “organoid” model derived from human tissue.
The model also reveals a possible biological target for treating GEJ cancers with a drug that the scientists have already shown can slow down or stop the growth of such tumors in mice.
Gastroenterologist Stephen Meltzer, M.D., the Harry and Betty Myerberg/Thomas R. Hendrix and American Cancer Society Clinical Research Professor of Medicine at the Johns Hopkins University School of Medicine and corresponding author of the study said, “Our model not only helps identify crucial changes happening during tumor growth at the GEJ but also establishes a strategy for future studies to help understand tumors of other organs.”
Scientists created this model using normal human biopsy tissue from patients receiving upper endoscopies. Organoids are three-dimensional collections of cells produced from stem cells that can mimic the traits of an organ or the functions of an organ, such as the production of particular cell types.
The scientists subsequently eliminated two crucial tumor suppressor genes (TP53 and CDKN2A) using the gene-editing technique clustered regularly interspaced palindromic repeats (CRISPR/Cas9) in the organoids. These genes were knocked out, which increased the cancerousness of the cells and gave them faster development and microscopic characteristics indicative of malignancy. Additionally, these modified organoids caused tumors in animals lacking immunity.
Platelet-activating factor was identified as a crucial upregulated lipid in GEJ organoids after the scientists discovered anomalies in a class of molecules (lipids) that have several different activities in addition to serving as energy stores. When platelets detect damaged blood vessels, they join together or form clots in the bloodstream, sometimes leading to clotting illnesses.
The study employed WEB2086, which prevented implanted GEJ organoid tumors from growing. The Food and Drug Administration-approved substance WEB2086, which is used to treat platelet disorders, inhibits platelet-activating factor receptors in my body.
Meltzer said, “More preclinical studies may be needed before using the compound for human patients, but that organoids may help advance such studies.”
“Combining organoids with this gene editing method [CRISPR/Cas9] is a potentially fruitful strategy for studying other human tumors in general.”
Journal Reference:
- Hua Zhao et al. Generation and multi-omic profiling of a TP53/CDKN2A double-knockout gastroesophageal junction organoid model. Science Translational Medicine. DOI: 10.1126/scitranslmed.abq6146