Alkali atoms are subtle promoters of catalytic reduction chemistry in vital chemical processes ranging from synthesizing agricultural chemicals to activating hydrocarbon feedstocks for industrial applications. They serve as a ready source of electrons that lower the activation energies and greatly enhance metal catalysts’ chemical activity for various industrially important reactions, including the water-gas shift reaction, the Fischer-Tropsch reaction, and Haber-Bosch ammonia synthesis.
Industrial production of NH3 has been performed by the Haber-Bosch process for more than 100 years, in which dissociation of N2 feedstock molecules promoted by alkali atom co-catalyst is thought to be the rate-limiting step.
The Haber-Bosch synthesis consumes 1% of the world’s total energy consumption and accounts for 1.4% of the global CO2 emissions. Therefore, the atomic-scale insights into the K atom-N2 molecule interactions on metal substrates, specifically the alkali atom promotion chemistry, have global significance.
Researchers at the University of Pittsburgh and theoretical collaborators at the University of Science and Technology of China have investigated the Haber-Bosch catalysis precursor at the atomic scale.
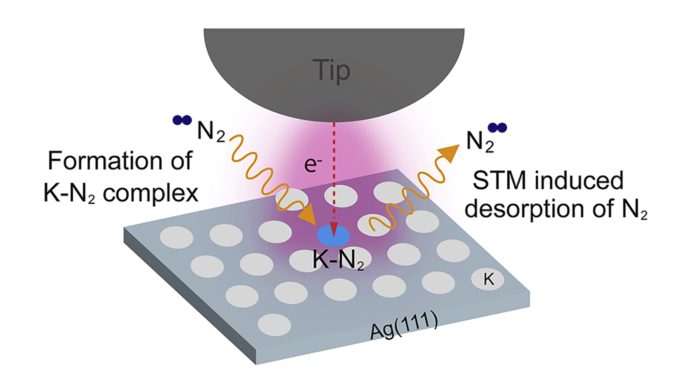
In a research article to be published in Cell Reports Physical Science on April 21, 2022, the researchers, led by Hrvoje Petek at the University of Pittsburgh, were able to directly observe at the atomic scale by scanning tunneling microscopy the N2 adsorption, their collective interactions, and tunneling electron-induced N2 desorption processes that are related to the alkali promotion of NH3 synthesis. The dominant pairwise interaction between the K and N2 is an electrostatic, two-center, Coulomb attraction, where charge transfer from K to N2 weakens the N2 molecule bond towards its dissociation in the Haber-Bosch synthesis. The K-N2 interactions interpreted through density functional theory are in agreement with the experimental observations.
Study Methodology
The correlated adsorbate K atom-N2 molecule interaction and tunneling electron-induced desorption related to the alkali promotion of Haber-Bosch ammonia synthesis are investigated on Ag(111) surface by STM imaging and DFT calculations.
Researchers show that N2 molecules interacting with K atoms condense into 1:1 K-N2 units that form (K-N2)x clusters (x = 1–4) at low N2 coverages. The K-N2 interactions interpreted through DFT calculations reproduce trends in atomic displacements and imaging contrast.
The attraction of weakly negatively charged N2 molecules with nearly completely ionized K atoms is electrostatic and only weakly dependent on the number of interacting species forming a complex. At higher N2 coverages, K-N2 complexes condense into featureless dark islands within the hexatic K atom overlayer, probably involving the complexation of K atoms by multiple N2 molecules leading to a higher condensation of the interacting species.
The condensation of such high-density K-N2 complex islands within the hexatic liquid K atom overlayer appears to be favored entropically because N2 molecules can complex K atoms in multiple nearly isoenergetic structures. The loss of K atom contrast in STM images of such clusters according to DFT calculations can be explained by changes in the K-N2 complex electronic structure in the vicinity of EF.
The strongly preferential condensation of K-N2 islands upon N2 molecule adsorption shows that to understand the catalytic processes of Haber-Bosch synthesis of ammonia, one must address the collective interactions between N2 molecules and their catalytic partners. The interfacial flow of charge into the K 4s orbital-derived unoccupied state, which causes Coulomb repulsion between K atoms and N2 molecules leading to N2 desorption, restores the uncomplexed K atom contrast.
Highlights
- Electrostatic interactions of K Haber-Bosch co-catalyst with N2 molecules
- STM measurements detect K atom displacement by N2 adsorption
- Collective K-N2 molecule interactions are evident
- DFT theory characterizes the N2 chemisorption on K/Ag(111) surface
“Our results reveal the fundamental interactions at the onset of correlated complexity that defines alkali atom promotion of catalytic chemistry.” Study quotes.
Journal Reference
- Chao Zhang, Linjie Chen, Jin Zhao, Hrvoje Petek; Imaging a Haber-Bosch catalysis precursor at the atomic scale. Cell Reports Physical Science, 3, 100865, May 18, 2022 DOI:10.1016/j.xcrp.2022.100865