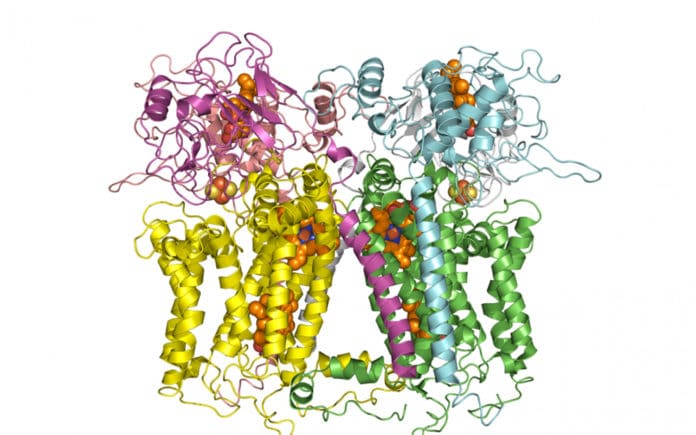
Mitochondria, a powerhouse of the cell, generates energy in the form of ATP. A pathway called electron transport chain- composed of five enzymes- together synthesizes ATP by a complicated movement of electrons and protons.
The third enzyme in this chain is cytochrome bc1 (or complex III), catalyzing proton pumping in response to electron transfer reactions. Mutations in complex III or Complex III (CIII) deficiency can cause several mitochondrial diseases.
A new study found that an exercise-intolerance-related mitochondrial disease mutation causes hydrogen bonding rearrangement that disturbs the normal functioning of the respiratory chain complex III.
Spectroscopic experiments have shown some distinct differences between the wild-type and the mutant enzyme.
The study was conducted by the Department of Physics, University of Helsinki, Finland, and Jagiellonian University, Krakow, Poland.
Scientists found that the results offer a novel interpretation of the experimental data after applying classical molecular dynamics simulations and density functional theory calculations on wild-type and mutant enzymes. It also yielded deeper mechanistic insights.
Vivek Sharma, Academy Research Fellow and Sigrid Jusélius Senior Researcher from the Department of Physics at the University of Helsinki, said, “These combined experimental-computational results provide detailed molecular insights; into how diseases may emerge by point mutations in mitochondrial enzymes and provide grounds for developing therapeutics of the future.”
Journal Reference:
- Patryk Kuleta et al., Hydrogen bonding rearrangement by a mitochondrial disease mutation in cytochrome bc1 perturbs heme by redox potential and spin state, Proceedings of the National Academy of Sciences (2021). DOI: 10.1073/pnas.2026169118