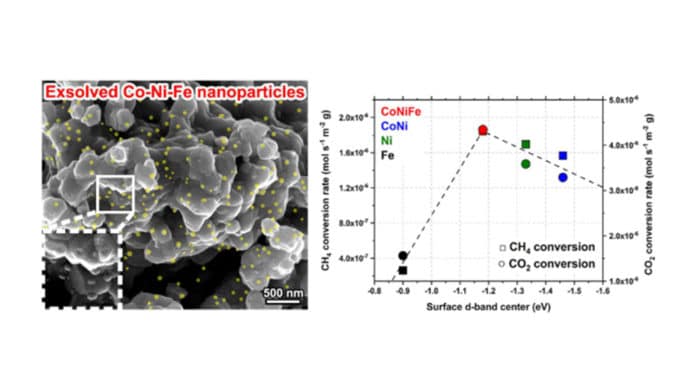
Scientists at UNIST have developed a ternary catalyst that converts greenhouse gases into hydrogen and carbon monoxide. The catalyst is composed of cobalt, nickel, and iron.
The dry reforming of methane (DRM) utilizes two ozone-harming substances, carbon dioxide (CO2) and methane (CH4), to create a syngas mixture of carbon monoxide (CO) and hydrogen (H2). Usually, nickel-based catalysts are the best options for the noble metals in the DRM response since they are cheap and dynamic. However, they become deactivated because of carbon deposition (coking), the accumulation of porous carbon materials, and the agglomeration of carbon particles.
Professor Guntae Kim in the School of Energy and Chemical Engineering at UNIST said, “This research, which focused on the development of a catalyst with enhanced activity and stability, will greatly contribute to the commercialization of the DRM technique.”
“The catalyst’s cobalt-nickel-iron alloy nanoparticles allow the generation of a larger number of exsolved nanoparticles, separated at critical temperatures, to cause more enhanced catalytic activity than the conventional catalyst’s nickel monometallic and cobalt-nickel bimetallic particles.”
“The activity and stability of a catalyst must be secured to produce syngas and hydrogen through DRM stably. The research team further noted that the exsolved catalyst exhibited exceptional stability, with continuous DRM operation for about 350 hours at a temperature above 750°C.”
Journal Reference:
- Sangwook Joo, Kyeounghak Kim, Ohhun Kwon, et al., Enhancing Thermocatalytic Activities by Upshifting the d-Band Center of Exsolved Co-Ni-Fe Ternary Alloy Nanoparticles for the Dry Reforming of Methane. DOI: 10.1002/anie.202101335