Moorella thermoacetica is a strictly anaerobic, endospore-forming, and metabolically versatile acetogenic bacterium capable of conserving energy by both autotrophic (acetogenesis) and heterotrophic (homoacetogenesis) modes of metabolism. Though, the bacterium does not work freely.
UC Berkeley analysts have figured out of it has an appetite for gold. Also, in return for this special treat, the bacterium has uncovered a more effective way of delivering solar fuels through artificial photosynthesis.
M. thermoacetica first made its introduction as the primary non-photosensitive bacterium to do artificial photosynthesis in an examination-driven by Peidong Yang, an educator in UC Berkeley’s College of Chemistry. By joining light-absorbing nanoparticles made of cadmium sulfide (CdS) to the bacterial membrane outside, the scientists turned M. thermoacetica into a little photosynthesis machine, changing over daylight and carbon dioxide into useful chemicals.
Now, scientists have discovered a better way to entice this CO2-hungry bacterium into being even more productive. By placing light-absorbing gold nanoclusters inside the bacterium, they have created a biohybrid system that produces a higher yield of chemical products than previously demonstrated. The research, funded by the National Institutes of Health, was published on Oct. 1 in Nature Nanotechnology.
To develop the model, scientists chose cadmium sulfide as the semiconductor for its ability to absorb visible light. But because cadmium sulfide is toxic to bacteria, the nanoparticles had to be attached to the cell membrane “extracellularly,” or outside the M. thermoacetica-CdS system.
Sunlight energizes every cadmium-sulfide nanoparticle into creating a charged molecule known as an electron. As these light-generated electrons travel through the bacterium, they associate with various proteins in a procedure known as “CO2 reduction,” setting off a course of reactions that inevitably transforms CO2 into acetate, a significant substance for making solar fuels.
However, inside the extracellular model, the electrons wind up collaborating with different chemicals that have no part in transforming CO2 into acetate. Furthermore, thus, a few electrons are lost and never achieve the enzymes.
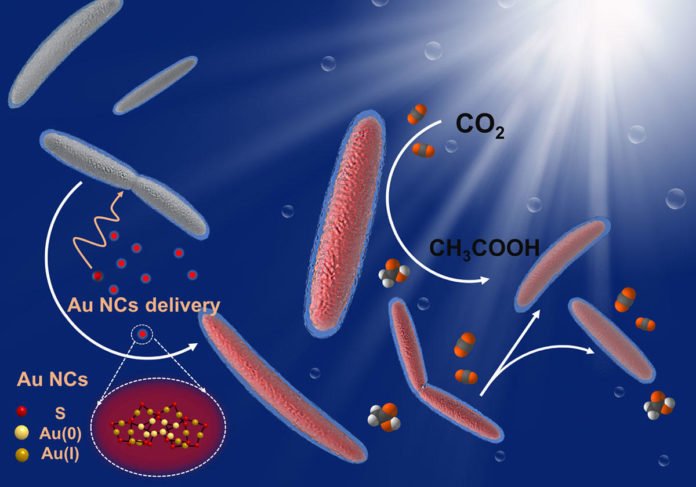
So to enhance what’s known as “quantum efficiency,” or the bacterium’s capacity to deliver acetate each time it picks up an electron, the specialists found another semiconductor: nanoclusters made of 22 gold atoms (Au22), a material that M. thermoacetica took an astonishing shine to.
Yang said, “We selected Au22 because it’s ideal for absorbing visible light and has the potential for driving the CO2 reduction process, but we weren’t sure whether it would be compatible with the bacteria. When we inspected them under the microscope, we discovered that the bacteria were loaded with these Au22 clusters – and were still happily alive.”
“By feeding bacteria with Au22 nanoclusters, we’ve effectively streamlined the electron transfer process for the CO2 reduction pathway inside the bacteria, as evidenced by a 2.86 percent quantum efficiency – or 33 percent more acetate produced within the M. thermoacetica-Au22 system than the CdS model.”
The magic gold nanocluster is the latest discovery coming out of Yang’s lab, which for the past six years has focused on using biohybrid nanostructures to convert CO2 into useful chemicals as part of an ongoing effort to find affordable, abundant resources for renewable fuels, and potential solutions to thwart the effects of climate change.