At the point when water encounters certain magnesium-and iron-rich mantle minerals, energizing things can occur. This process is also known as serpentinization, makes hydrogen and, at the seafloor, can prompt the development of hydrocarbons and little natural mixes.
The results of serpentinization support hydrothermal vent ecosystems and may have made conditions lenient for the root of life. Normally, researchers are keen on taking in the better subtleties of how this procedure jumps out at draw nearer to characterizing when and how life started.
Scientists performed computer simulations and spectroscopic analyses of fine-scale interactions between water and a type of olivine, a major rock-forming mineral in the upper mantle.
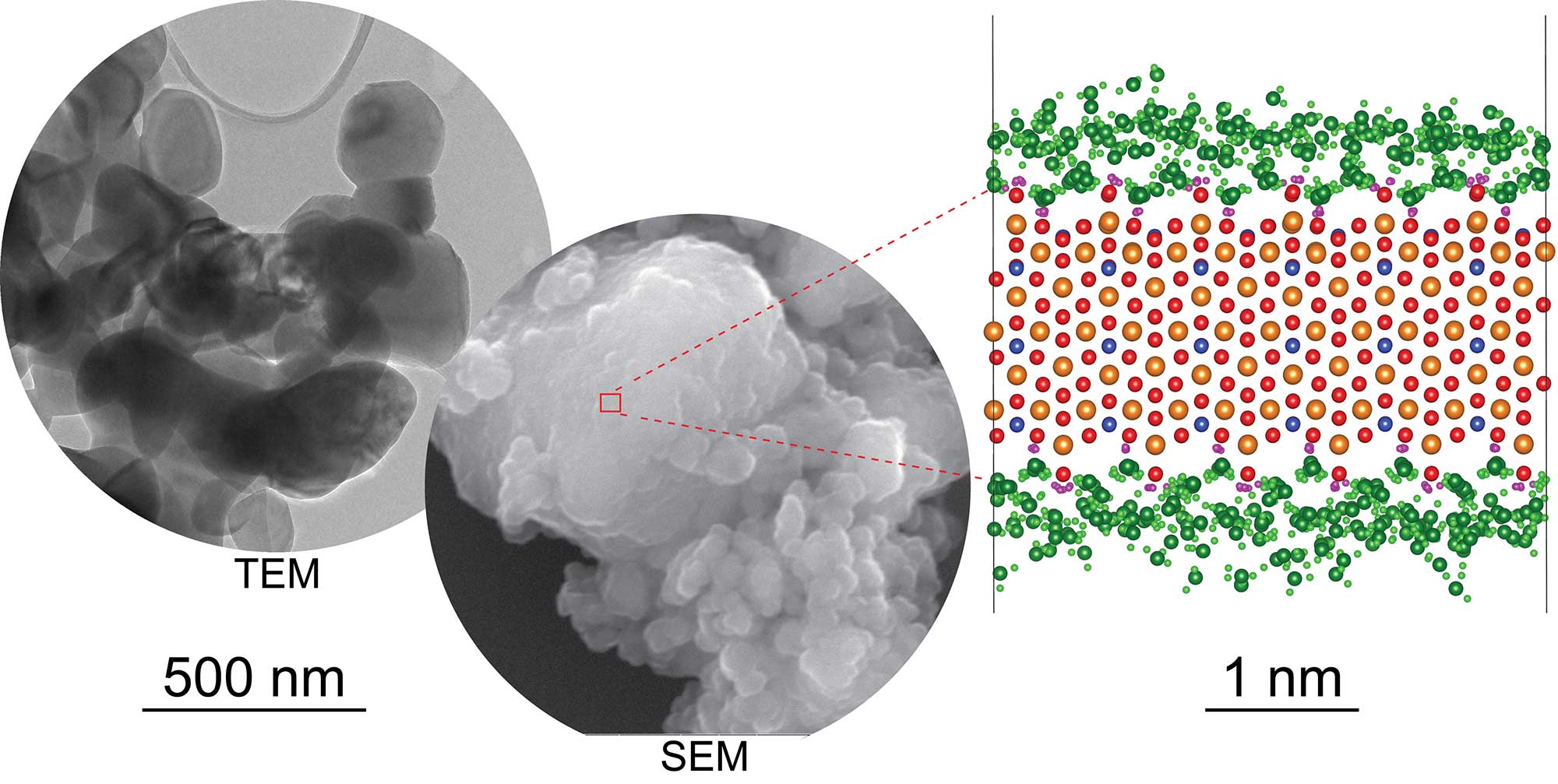
The outcomes suggest that water forms three particular layers when it interacts with the olivine. The recreations and estimations supplement one another, enhancing our comprehension of the structure and dynamics of water-olivine connections, and establish a foundation for future research on serpentinization.
DCO member Tingting Liu said, “Olivine is one of the most widespread silicates on Earth. Its interaction with water in nature is ubiquitous. This work is the first step to understand the mechanism of adsorption and water dynamics on the surface of olivine. It describes the first step in the serpentinization process.”
Through, computer simulation, scientists observed the interaction of water with a form of olivine called forsterite and the physical movements of molecules during the interaction.
The simulations predicted that the water forms three distinct layers as it encounters the forsterite surface. The water molecules closest to the forsterite surface stick, creating an extremely dense and almost immobile layer. The second and third layers of water molecules are less dense, and molecules can move between the two layers. They found that the top layer contributes the major mobility of the water-olivine interface.
Later on, scientists used quasi-elastic neutron scattering (QENS) for the detection of water molecules on the forsterite surface. They shoot a beam of neutrons at a sample and then detect the neutrons scattered by the sample.
The scattering event encodes data about the example. They utilized nanopowdered forsterite synthesized by partners at Oak Ridge National Laboratory and hydrated with water vapor. The scientists repeated this analysis on three distinctive neutron dispersing instruments, with the goal that they could distinguish the water’s dynamics over timescales of 1 picosecond to 1 nanosecond.
The study shows a complete picture of water-olivine interaction. It could help scientists understand the capacity of basalt minerals at the seafloor to sequester carbon dioxide from the surface and the formation of hydrocarbons through chemical reactions independent of life, in submarine environments.
The study is published in Physical Chemistry Chemical Physics. The co-authors of the study include Tingting Liu, Siddharth Gautam, David Cole (all at Ohio State University, USA), and Eugene Mamantov (Oak Ridge National Laboratory, USA).
