Scientists for the first ever time have measured how diamonds grow at the atomic level i.e., from seed. Their discovery enlightens how nucleation continues not simply in diamonds, but rather in the environment, in silicon gems utilized for computer chips and even in proteins that cluster together in neurological infections.
Nicholas Melosh, a professor at Stanford University said, “Nucleation growth is a core tenet of materials science, and there’s a theory and a formula that describes how this happens in every textbook. It’s how we describe going from one material phase to another, for example from liquid water to ice.”
“But interestingly, despite the widespread use of this process everywhere, the theory behind it had never been tested experimentally, because observing how crystal growth starts from atomic-scale seeds is extremely difficult.”
According to scientists, the theory overestimates how much energy it takes to kick off the nucleation process. Thus, they have developed a method that can potentially introduce the theory to reality. But the problem is the method until now, tested on a large scale.
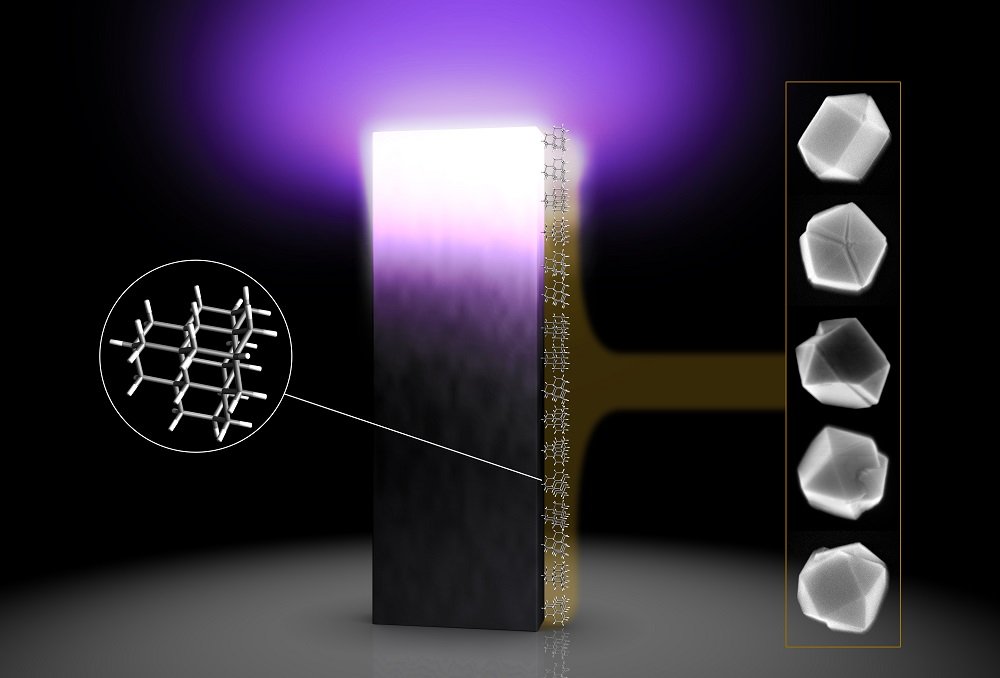
In order to test it on small scale, scientists at the SLAC-Stanford moved towards diamondoids, the tiniest possible bits of the diamond. These bits are the focal point of a DOE-supported program at SLAC and Stanford where normally happening diamondoids are isolated from petroleum fluids, arranged by size and shape and contemplated.
Ongoing trials recommend they could be utilized as Lego-like squares for gathering nanowires or “molecular anvils” for activating compound responses, in addition to other things.
Matthew Gebbie, Stanford researcher, who is interested in the chemistry of interfaces – places where one phase of matter encounters another, for instance, the boundary between air and water, suggests that ‘interfaces are incredibly important in growing diamonds with a process called CVD, or chemical vapor deposition.’
He said, “What I’m excited about is understanding how size and shape and molecular structure influence the properties of materials that are important for emerging technologies. That includes nanoscale diamonds for use in sensors and in quantum computing. We need to make them reliably and with consistently high quality.”
In order to grow the diamond with CVD, scientists crushed diamond into tiny bits and seeded them on the surface. They later disclose them to plasma, which composed of hydrogen and carbon, two essential elements needed to form a diamond. The crystals that grow around the seeds eventually get big enough to count under a microscope.
This experiment gives scientists a much finer level of control over this process. Although they’re too small to see directly, even with the most powerful microscopes, they can be precisely sorted according to the number of carbon atoms they contain and then chemically attached to the surface of a silicon wafer so they’re pinned in place while being exposed to a plasma.
What scientists found, is the crystal growth really took off with seeds that contain at least 26 carbon atoms. Moreover, they were even able to estimate the energy barrier of diamondoid particles.
Gebbie said, “It was thought that this barrier must be like a gigantic mountain that the carbon atoms should not be able to cross – and, in fact, for decades there’s been an open question of why we could even make diamonds in the first place. What we found was more like a mild hill.”
“This is really fundamental research, but at the end of the day, what we’re really excited about and driving for is a predictable and reliable way to make diamond nanomaterials. Now that we’ve developed the underlying scientific knowledge needed to do that, we’ll be looking for ways to put these diamond nanomaterials to practical use.”
The study is published in the Proceedings of the National Academy of Sciences.
