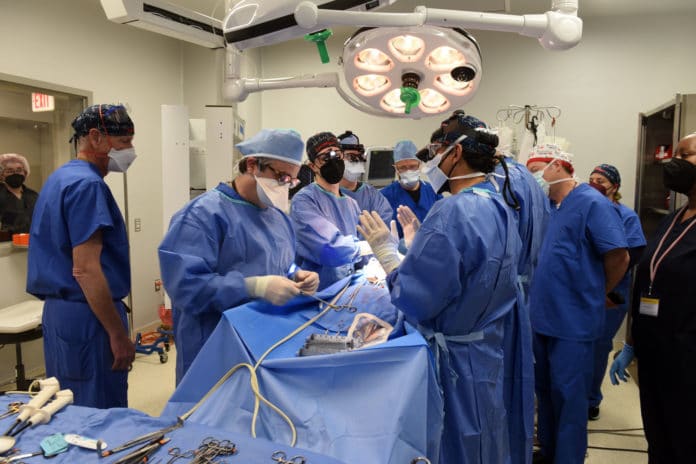
For the first time, scientists successfully performed the first pig-to-human heart transplant. A 57-year-old patient with terminal heart disease received a successful transplant of a genetically-modified pig heart. And the exciting fact is, the patient David Bennett, a Maryland resident, is doing well three days later.
This first-of-its-kind transplant was performed by scientists and clinicians at the University of Maryland School of Medicine (UMSOM) faculty at the University of Maryland Medical Center (UMMC).
Bartley P. Griffith, MD, who surgically transplanted the pig heart into the patient, said, “This was a breakthrough surgery and brought us one step closer to solving the organ shortage crisis. There are not enough donor human hearts available to meet the long list of potential recipients.”
“We are proceeding cautiously, but we are also optimistic that this first-in-the-world surgery will provide an important new option for patients in the future.”
Muhammad M. Mohiuddin, MD, Professor of Surgery at UMSOM, said, “This is the culmination of years of highly complicated research to hone this technique in animals with survival times that have reached beyond nine months. The FDA used our data and data on the experimental pig to authorize the transplant in an end-stage heart disease patient who had no other treatment options.”
“The successful procedure provided valuable information to help the medical community improve this potentially life-saving method in future patients.”
Transplanting animal organs to humans is known as xenotransplantation. This procedure could potentially save thousands of lives. But, it has a unique set of risks, including the possibility of triggering a dangerous immune response. These responses can trigger an immediate rejection of the organ with a potentially deadly outcome to the patient.
The procedure was first performed in the 1980s on an infant born with a fatal heart condition. The infant received a baboon heart transplant and died within a month of the procedure due to the immune system‘s rejection of the foreign heart.
However, for many years, pig heart valves have been used successfully for replacing valves in humans.
Before performing this surgery, Mr. Bennett was fully informed of the procedure’s risks.
Mr. Bennett had life-threatening arrhythmia. To remain alive, he was connected to a heart-lung bypass machine called extracorporeal membrane oxygenation (ECMO).
He had been deemed ineligible for a conventional heart transplant at UMMC and several other leading transplant centers that reviewed his medical records. On New Year’s Eve, the U.S. Food and Drug Administration granted emergency authorization for the surgery.
The genetically-modified pig was provided by a Blacksburg-based regenerative medicine company- Revivicor. The Revivocor delivered the pig to the xenotransplantation laboratory at UMSOM. On the morning of the transplant surgery, the surgical team removed the pig’s heart and placed it in the XVIVO Heart Box, a perfusion device.
In addition to conventional anti-rejection drugs, the team used a new drug- an experimental compound made by Kiniksa Pharmaceuticals.
E. Albert Reece, MD, Ph.D., MBA, Executive Vice President for Medical Affairs, UM Baltimore, said, “This unprecedented and historic procedure highlights the importance of translational research, which lays the groundwork for patients to benefit in the future. It is the culmination of our longstanding commitment to discovery and innovation in our xenotransplantation program.”
John Z. and Akiko K. Bowers, Distinguished Professor and Dean, University of Maryland School of Medicine, said, “Our transplant surgeon-scientists are among the most talented in the country and are helping to bring the promise of xenotransplantation to fruition. We hope it will one day become a standard of care for patients in need of organ transplants. As has happened throughout our history, the University of Maryland School of Medicine continues to address the most complex medical and scientific problems.”
Bruce Jarrell, MD, President of the University of Maryland, Baltimore, who himself is a transplant surgeon, recalled: “Dr. Griffith and I began as organ transplant surgeons when it was in its infancy. Back then, it was the dream of every transplant surgeon, myself included, to achieve xenotransplantation. It is now personally gratifying to see this long-sought goal clearly in view. It is a spectacular achievement.”
Bert W. O’Malley, MD, President and CEO, University of Maryland Medical Center, said, “This is truly a historic, monumental step forward. While we have long been at the forefront of research driving progress toward the promise of xenotransplantation as a viable solution to the organ crisis, many believed this breakthrough would be well into the future.”
“I couldn’t be more proud to say the future is now. Our skilled team of UMMC and UMSOM physician-scientists will continue to advance and adapt medical discovery for patient care that could offer a lifeline for more patients in dire need.”
Mohan Suntha, MD, MBA, President and CEO, University of Maryland Medical System, added: “The University of Maryland Medical System is committed to working with our University of Maryland School of Medicine partners to explore, research, and in many cases implement the innovations in patient care that make it possible to improve quality of life and save lives. We appreciate the tremendous courage of this live recipient, who has made an extraordinary decision to participate in this groundbreaking procedure to not only potentially extend his own life but also for the future benefit of others.”
The team knocked out three genes in donor pigs responsible for humans’ rapid antibody-mediated rejection of pig organs. Six human genes responsible for the immune acceptance of the pig heart were inserted into the genome. They again knocked one additional gene in the pig to prevent excessive growth of the pig heart tissue. Overall, ten gene edits were made in the donor pig.
David Ayres, Ph.D., Chief Scientific Officer of Revivicor, Inc, said, “We are thrilled to support the world-class team of transplant surgeons led by Dr. Griffith and Dr. Mohiuddin at the University of Maryland School of Medicine. This transplant is groundbreaking and is another step in investigating Xeno organs for human use.”
Christine Lau, MD, MBA the Dr. Robert W. Buxton Professor and Chair of the Department of Surgery at UMSOM and Surgeon-in-Chief at UMMC, said, “As a cardiothoracic surgeon who does lung transplants, this is an amazing moment in the history of our field. Decades of research here at Maryland and elsewhere have gone into this achievement. This can revolutionize the field of transplantation by eventually eliminating the organ shortage crisis. This is a continuation of steps to making xenotransplantation a life-saving reality for patients in need.”
Daniel G Maluf, MD, FAST, Professor of Surgery and Medicine at UMSOM and Director, UMSOM’s Program in Transplantation, added: “This is a breakthrough for the field of organ transplantation and medicine,” he said. “This event is the final achievement of years of research and testing from our multidisciplinary team led by Dr. Griffith and Dr. Mohiuddin and represents a beginning of a new era in the field of organ transplantation medicine. I am proud of our team’s incredible achievement.”
Peter Rock, MD, MBA, the Dr. Martin Helrich Chair and Professor in the Department of Anesthesiology at UMSOM, said: “We carefully considered the unique needs of this patient in preparing him for surgery and the intricacies involved in modifying our anesthetic techniques for this xenotransplant procedure. Our planning paid off, and the surgery could not have gone better thanks to the herculean efforts of the medical team involved in this landmark event.”