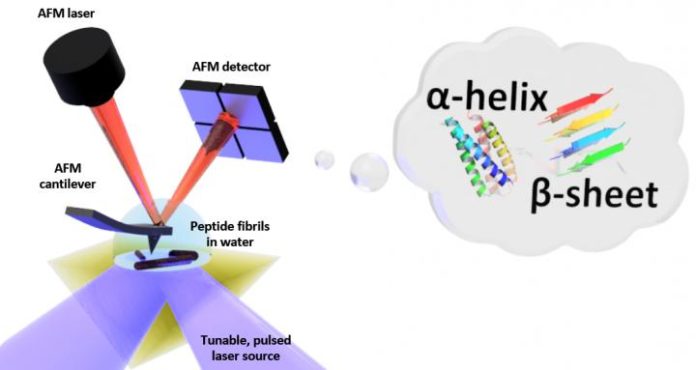
Scientists have previously developed a spectroscopy technique to measure a material’s topography and chemical composition with nanometer-scale. Now NIST scientists came up with little improvement to this technique. For the first time, they have measured at the nanometer scale the characteristic patterns of folds that give proteins their three-dimensional shape in the water.
This new upgradation in the technique is expected to help scientists gain insights into the behavior of biomolecules in watery environments similar to those in cells. The insights could potentially deepen our understanding of major diseases, including Alzheimer’s, that are related to “mistakes” in protein folding.
Proteins in blood fold into precise patterns leading to helices, sheets and other shapes that give proteins their three-dimensional structure. Such types of shapes enable proteins to carry oxygen, fend off harmful bacteria and perform other essential tasks in the body.
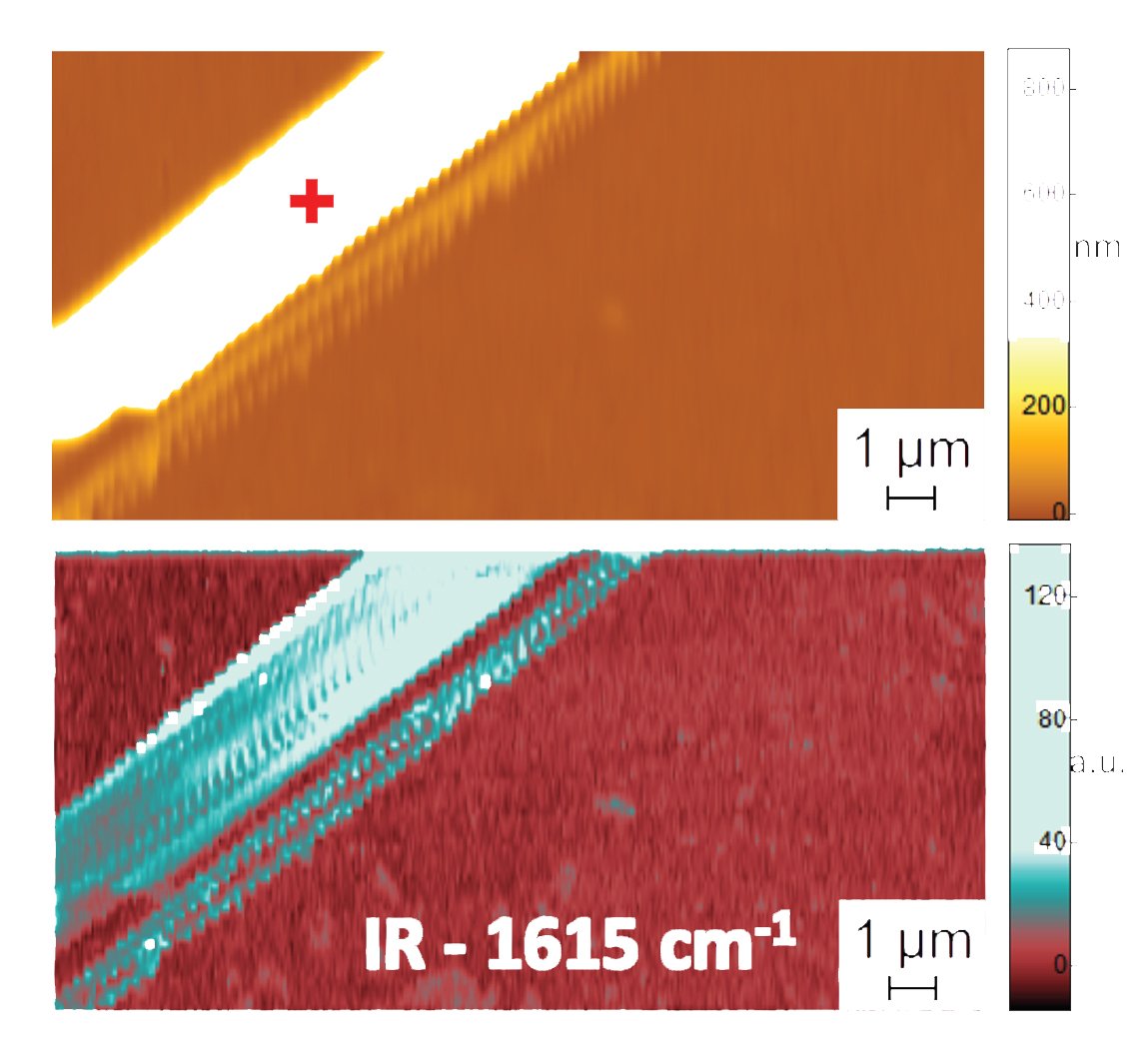
In order to understand, how this folding happens, scientists primarily need to understand the detailed arrangement of chains of amino acids that are shorter and simpler than proteins—called peptides—and how they fold, assemble and rotate to create a variety of shapes or conformations.
Scientists decided to study the proteins and peptides within water because that environment closely approximates the conditions inside living cells.
Previously demonstrated techniques lack the fine spatial resolution to study the tiny and diverse assemblies of properly folded and misfolded proteins. In addition, these techniques don’t work well in an aqueous environment because water strongly absorbs infrared light, confounding the analysis.
Water had additionally postured serious difficulties for a spearheading system, known as photo-thermal induced resonance (PTIR), that empowered analysts to analyze peptide structure and compliance in air with nanoscale determination.
Scientists have now demonstrated that PTIR can be adapted to obtain conformational structure at the nanoscale in water using two chemically similar peptides known as diphenylalanine and Boc-diphenylalanine. Diphenylalanine is related to beta-amyloid, a sticky, larger peptide linked to Alzheimer’s disease.
Georg Ramer of NIST and the University of Maryland in College Park said, “PTIR is a powerful technique that had already shown promise for the study of biological systems, but the possibility to use this with samples in a liquid environment will greatly improve its use in this area.”
PTIR determines the chemical composition of materials with nanoscale determination by consolidating an atomic force microscope (AFM) with light from an infrared laser that works over a scope of wavelengths. These wavelengths of infrared light that are then consumed by the sample are much the same as a molecular fingerprint, uncovering its chemical compound.
At each site on the sample where infrared is consumed, the material warms up, making it quickly, yet somewhat, grow. The development is recognized, with the sharp tip of the AFM projecting from a cantilever, which wavers like a diving board each time the example grows. The all the more light that is consumed by the sample, the more noteworthy its development and the larger the strength, or amplitude, of the oscillations.
But using PTIR in water seems problematic as it absorbs infrared light and thus generates an absorption signal that can interfere with efforts to discern the sample’s chemical structure.
In order to prevent light absorption, scientists placed a prism between the laser and the sample. The prism restricts the infrared light to the sample’s surface and thus reduce the amount that could leak out and interact with the water.
Scientists also used a laser that operates at frequencies up to 2,000 kilohertz. The laser addresses the damping problem and enabled the researchers to match the frequency of the laser pulses to one of the higher frequencies at which the cantilever oscillates.
To demonstrate the accuracy of their method, the team compared PTIR measurements of diphenylalanine and other peptide samples in two environments: water and air. (The peptides folded similarly in both mediums, making it easier to perform the comparison.) Remarkably, the scientists achieved similar spatial resolution and contrast in water and air, demonstrating for the first time that measurements in a water environment can be performed accurately, revealing the precise conformation of peptides with nanoscale resolution.
The paper describing the technique is published in the ACS Nano.