All-solid-state batteries are a new kind of Li-ion battery and have been appeared to be possibly more secure and more steady energy-storing gadgets with higher energy densities. In any case, the utilization of such batteries is restricted because of a noteworthy detriment: their opposition at the electrode/solid electrolyte interface is too high, impeding quick charging and releasing.
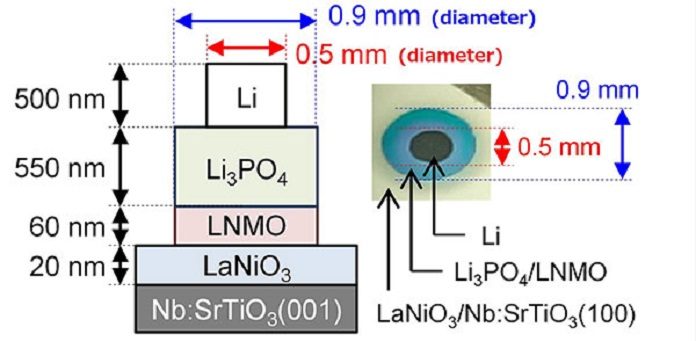
Scientists at Tokyo Tech have addressed this major issue by developing batteries with a low resistance at their electrode/solid electrolyte interface. They have developed all-solid-state batteries with extremely low interface resistance using Li(Ni0.5Mn1.5)O4 (LNMO), by fabricating and measuring their batteries under ultrahigh vacuum conditions.
After manufacture, the electrochemical properties of these batteries were described to reveal insight into Li-ion dissemination around the interface. X-ray diffraction and Raman spectroscopy were utilized in analyzing the crystal structure of the thin movies involving the batteries.
Unconstrained relocation of Li ions was found to happen from the Li3PO4 layer to the LNMO layer, changing over a large portion of the LNMO to L2NMO at the Li3PO4/LNMO interface. The invert relocation happens amid the underlying charging procedure to recover LNMO.
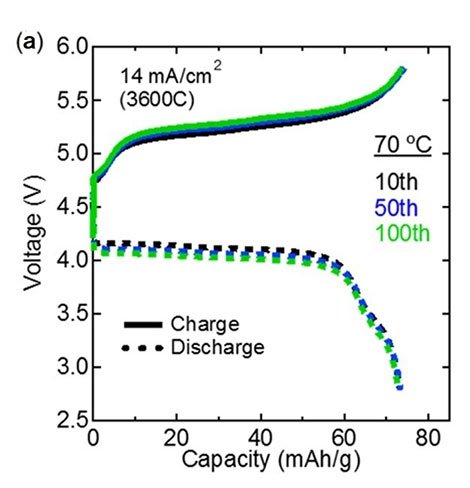
The (a) charge-discharge curves and the (b) cycling performance plot show that the performance of the fabricated all-solid-state batteries did not degrade after repeated use, demonstrating their excellent stability and the total reversibility of the reactions involved in charging/discharging.
The opposition of this interface, confirmed utilizing electrochemical impedance spectroscopy, was 7.6 Ωcm2, two orders of magnitude smaller than that of past LMNO-based all-solid-state batteries and significantly smaller than that of liquid electrolyte-based Li-ion batteries utilizing LNMO.
These batteries likewise showed quick charging and releasing, figuring out how to charge/release a large portion of the battery within a moment. Besides, the cyclability of the battery was likewise great, demonstrating no corruption in execution even after 100 charge/release cycles.
Li(Ni0.5Mn1.5)O4 is a promising material to increase the energy density of a battery because the material provides us with a higher voltage. The research team hopes that these results will facilitate the development of high-performance all-solid-state batteries, which could revolutionize modern portable electronic devices and electric cars.
The study is published in ACS Applied Materials & Interfaces.